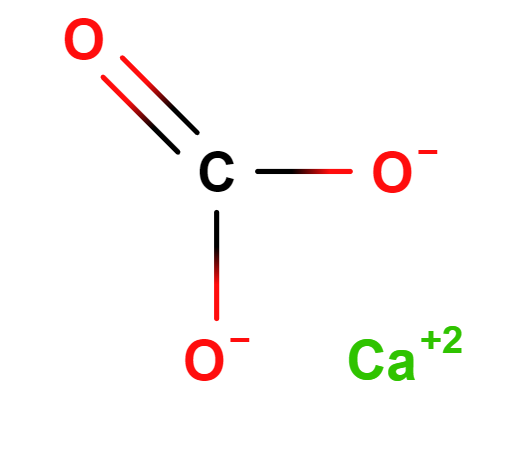
Calcite or Calcium carbonate, an inorganic compound, is one of the most common minerals found in rock agglomerates throughout the world and consists mainly of: calcium, carbon, oxygen. It is the primary component of eggshells, snails, pearls and marine organisms. Calcium carbonate is the active ingredient in agricultural lime and is created when calcium ions in hard water react with carbonate ions to create limescale.
The name "calcium carbonate" is derived from its chemical composition: one calcium ion combined with one carbonate ion.
Calcium carbonate is not typically synthesized in a laboratory or industrial setting because it is readily available in nature. However, it can be synthesized by mixing solutions of calcium ions and carbonate ions. The resulting precipitate is calcium carbonate.
Here is a simple example of how this can be done:
- Preparation of solutions. A solution of calcium chloride (CaCl2) and another solution of sodium carbonate (Na2CO3). These are the sources of the calcium ions and carbonate ions, respectively.
- Mixing of solutions. A sodium carbonate solution is added to the calcium chloride solution while the compounds are mixed.
- Formation of precipitate. As the solutions mix, a precipitate of calcium carbonate forms. The reaction is: CaCl2 + Na2CO3 -> CaCO3 + 2NaCl.
- Isolation of product. The calcium carbonate precipitate can be collected by filtration, then washed and dried to obtain the pure compound.
It appears in the form of a white powder insoluble in water colorless, odorless and tasteless.

What it is used for and where
Food
Ingredient included in the list of European food additives as dye E170.
Constructions (cement, plasters, asphalt).
The use of calcium carbonate precipitate protect concrete surface against the ingress of harmful external agents (1).
Medical
Antacid. Calcium carbonate is widely used as an antacid. It helps neutralize acid in the stomach, providing relief from indigestion symptoms.
Calcium Supply. Calcium carbonate is a common source of calcium in calcium supplement formulations. Calcium is an essential nutrient for bone and teeth health.
Binder. Calcium carbonate can be used as a binder in tablets. It helps hold the ingredients in the tablet together.
Diluent. Calcium carbonate can be used as a diluent in tablets. It increases the volume of the tablet, making it easier to handle and swallow.
Coloring Agent. Calcium carbonate is sometimes used as a white coloring agent in tablets.
The synthesised biobased Calcium carbonate nanocrystals had demonstrated to be an effective carrier for delivery of anticancer drug doxorubicin (DOX). Findings suggest that Calcium carbonate nanocrystals hold tremendous promise in the areas of controlled drug delivery and targeted cancer therapy (2).
Dentin hypersensitivity reduction in adults with a clinical diagnosis of dentin hypersensitivity (3).and indicated as a treatment in cases of calcium deficiency in elderly subjects and in combination with therapies for osteoporosis (4). Calcium carbonate is able to increase calcium levels and neutralise plaque acids (5).
Cosmetics
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Safety. It is an ingredient that has no particular health warnings and can therefore be used in all cosmetic products.
and is widely used by industries:
- Adhesives, sealants
- Rugs
- Environment (process of desulfurization of combustion gases)
- Fertilizers
- Food and pharmaceutical
- Glass and ceramics
- Products for the home
- Paints and surface coatings
- Paper
- Plastics and composites
- Rubber and elastomers
For more information:
Calcium carbonate studies
Typical commercial product characteristics Calcium carbonate
| Appearance | White powder |
| pH | 9.0-10.5 |
Boiling Point
| 333.6ºC at 760mmHg |
Melting Point
| 825°C |
Flash Point
| 197ºC |
| Density | 2.93 g/mL at 25 °C(lit.) |
| Refraction Index | 1.6583 |
| PSA | 63.19000 |
HCL insoluble content
| ≤0.20% |
Volatile content below 105℃
| ≤1.00 |
Free Alkali
| ≤0.10% |
Whiteness degree
| ≥90% |
Oil Absorption
| 50-60ml/g |
Sedimentation volume
| ≥2.2-2.8 ml/g |
| Fe | ≤0.12% |
| Mn | ≤0.01% |
Average Particle
| 0.5-15um |
| Mesh | 400/800/1000/1250/1500 |
| Safety |  |
- Molecular Formula CCaO3 CaCO3
- Molecular Weight 100.086 g/mol
- Exact Mass 101.962982
- CAS 471-34-1
- UNII H0G9379FGK
- EC Number 207-439-9
- DSSTox Substance ID DTXSID3036238 DTXSID9050486 DTXSID90109374 DTXSID80163829
- IUPAC calcium;carbonate
- InChI=1S/CH2O3.Ca/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2
- InChl Key VTYYLEPIZMXCLO-UHFFFAOYSA-L
- SMILES C(=O)([O-])[O-].[Ca+2]
- MDL number MFCD00010906
- PubChem Substance ID 329749202
- ChEBI 3311
- ICSC 1193
- RXCUI 1897
- RTECS EV9580000 FF9335000
- Beilstein 8008338
- NCI C332
Synonyms :
- CI 77220
- Calcite (Ca(Co3))
- Aragonite
- Cupric carbonate
- C50 (carbonate)
- Carbonic acid, calcium salt (1:1)
References____________________________________________________________________
(1) Van Tittelboom K, De Belie N, De Muynck W, Verstraete W. 2010. Use of bacteria to repair cracks in concrete. Cem. Concr. Res. 40:157–166. 10.1016/j.cemconres.2009.08.025
(2) Shafiu Kamba A, Ismail M, Tengku Ibrahim TA, Zakaria ZA. A pH-sensitive, biobased calcium carbonate aragonite nanocrystal as a novel anticancer delivery system. Biomed Res Int. 2013;2013:587451. doi: 10.1155/2013/587451.
(3) Collins JR, Richardson D, Sotero K, Mateo LR, Mauriz I. Beneficial effects of an arginine-calcium carbonate desensitizing paste for treatment of dentin hypersensitivity. Am J Dent. 2013 Apr;26(2):63-7.
(4) Wang J, Tao S, Jin X, Song Y, Zhou W, Lou H, Zhao R, Wang C, Hu F, Yuan H. Calcium Supplement by Tetracycline guided amorphous Calcium Carbonate potentiates Osteoblast promotion for Synergetic Osteoporosis Therapy. Theranostics. 2020 Jul 9;10(19):8591-8605. doi: 10.7150/thno.45142.
(5) Lynch RJ, ten Cate JM. The anti-caries efficacy of calcium carbonate-based fluoride toothpastes. Int Dent J. 2005;55(3 Suppl 1):175-8. doi: 10.1111/j.1875-595x.2005.tb00055.x.
![]() Calcite
Calcite