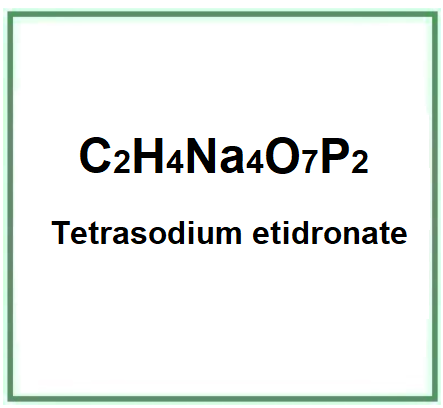
![]() Tetrasodium etidronate
Tetrasodium etidronate
Rating : 5
Cons:
Possible eye irritant (1)Tetrasodium Etidronate is a chemical compound.Decomposition of the name and function of the componentsTetrasodium - Indicates the presence of four sodium ions. These ions are vital for the compound's water solubility.Etidronate - Derived from etidronic acid, a phosphonic acid. Etidronic acid is the backbone of the compound, responsible for its ... (Read the full Tiiip)
10 pts from Qwerty
| Evaluate | Where is this found? |
| "Descrizione" about Tetrasodium etidronate Review Consensus 10 by Qwerty (3763 pt) | 2024-Jan-03 15:03 |
Tetrasodium Etidronate is a chemical compound.Decomposition of the name and function of the componentsTetrasodium - Indicates the presence of four sodium ions. These ions are vital for the compound ...
| Read the full Tiiip | (Send your comment) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2023-08-01 10:17:53 | Chemical Risk: |