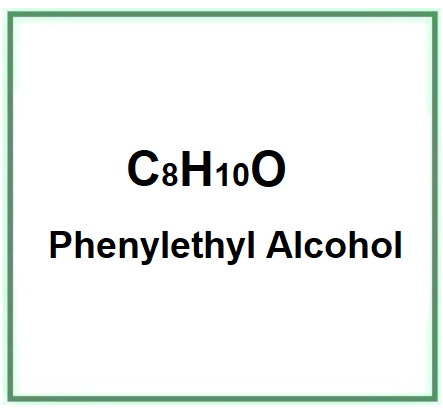
Phenylethyl Alcohol is a chemical compound, a primary alcohol member of the structural group Aryl Alkyl Alcohols.
The name defines the structure of the molecule
- "Phenyl" refers to the functional group derived from benzene when one hydrogen atom is removed. It has the formula C₆H₅.
- "ethyl" refers to the alkyl group with two carbon atoms, having the formula CH₂CH₃.
- "Alcohol" indicates the presence of a hydroxyl group (-OH) in the molecule.
Description of raw materials used in production
- Ethylene - A carbon source.
- Benzene - Serves as the aromatic core.
Step-by-step summary of industrial chemical synthesis process.
- Alkylation - Benzene and ethylene undergo alkylation to produce ethylbenzene.
- Oxidation - Ethylbenzene is then oxidized to form phenylacetaldehyde.
- Reduction - Phenylacetaldehyde is reduced using hydrogen in the presence of a catalyst to form phenylethyl alcohol.
- Purification - The crude phenylethyl alcohol is purified via processes such as distillation.
- Quality Control - A quality check is done on the phenylethyl alcohol to ensure it meets the desired specifications.
It appears as a colorless liquid

What it is for and where
Cosmetics
Fragrance. It plays a decisive and important role in the formulation of cosmetic products as it provides the possibility of enhancing, masking or adding fragrance to the final product, increasing its marketability. It is able to create a perceptible pleasant odour, masking a bad smell. The consumer always expects to find a pleasant or distinctive scent in a cosmetic product.
Safety
Studies conducted to compare the dermal absorption, plasma pharmacokinetics, and excretion of phenylethyl alcohol (PEA) by pregnant and nonpregnant rats, rabbits, and humans under normal conditions of fragrance use find that PEA is not a developmental toxicity hazard to humans (1).
Commercial applications
Perfumes and Fragrances. Used for its pleasant floral fragrance in perfumes, cosmetics, and personal care products.
Preservative. Added to cosmetic and skincare products to prevent microbial growth.
Solvent. Employed in various industrial applications as a solvent for different chemical substances.
Flavoring. Can be used in small amounts as a flavoring in food and beverages.
Research. Used in microbiology laboratories as a selective component in culture media to inhibit the growth of certain microorganisms.
- Molecular Formula C8H10O
- Molecular Weight 122.16 g/mol
- CAS 60-12-8
- UNII ML9LGA7468
- EC Number 200-456-2
- DTXSID9026342
- Nikkaji J1.924K
References_____________________________________________________________________
(1) Politano VT, Diener RM, Christian MS, Hawkins DR, Ritacco G, Api AM. The pharmacokinetics of phenylethyl alcohol (PEA): safety evaluation comparisons in rats, rabbits, and humans. Int J Toxicol. 2013 Jan-Feb;32(1):39-47. doi: 10.1177/1091581812471688.
Abstract. The present studies were conducted to compare the dermal absorption, plasma pharmacokinetics, and excretion of phenylethyl alcohol (PEA) by pregnant and nonpregnant rats, rabbits, and humans. The PEA is a natural fragrance material that is widely used in perfumes, soaps, and lotions and is a major ingredient of natural rose oil. Following dermal (430, 700, or 1400 mg/kg body weight [bw]), gavage (430 mg/kg bw), or dietary (430 mg/kg bw) administration of PEA to rats, plasma concentrations of PEA were found to be low regardless of the route of administration. The plasma concentrations of phenylacetic acid (PAA, the major metabolite of PEA) greatly exceeded the concentrations of PEA and were highest after gavage, followed by dermal then dietary administration. Absorption, distribution, metabolism, and excretion were compared following topical application of ¹⁴C-labeled PEA to rats, rabbits, and humans (specific activities of dosing solutions: 58-580, 164, and 50 µCi/mL, respectively). In rabbits, the plasma concentration-time profile for PAA was markedly prolonged compared to rats or humans. In humans, only 7.6% of the applied dose of PEA was absorbed, versus 77% in rats and 50% in rabbits. Based on a human dermal systemic exposure of 0.3 mg/kg per day from the use of multiple consumer personal care products containing PEA, a rat dermal no observed adverse effect level of 70 mg/kg per day, and the percentage of dose absorbed in humans, the margin of safety exceeds 2600 concluding that, under normal fragrance use conditions, PEA is not a developmental toxicity hazard for humans.
(2) Scognamiglio J, Jones L, Letizia CS, Api AM. Fragrance material review on phenylethyl alcohol. Food Chem Toxicol. 2012 Sep;50 Suppl 2:S224-39. doi: 10.1016/j.fct.2011.10.028.
![]() Phenylethyl alcohol
Phenylethyl alcohol