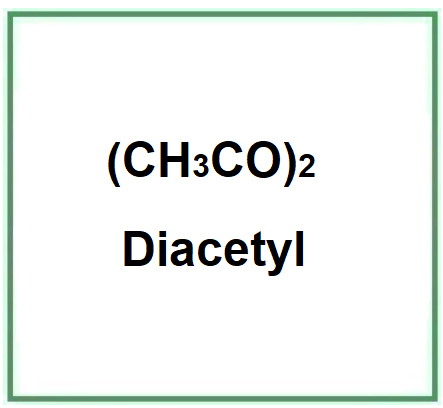
Diacetyl (2,3 butanedione) is an organic chemical compound, a volatile vicinal diketone.
The name describes the structure of the molecule
- "Di" indicates the presence of two functional groups in the molecule.
- "Acetyl" refers to the acetyl functional group, which is derived from acetic acid.
Description of raw materials used in production
- Bacterial culture - For instance, Lactobacillus bacteria used in fermentation.
- Sugary substrates - Such as glucose or maltose.
Step-by-step summary of industrial chemical synthesis process
- Substrate Preparation - Sugary substrates are prepared and solubilized in water.
- Inoculation - The bacterial culture is introduced into the sugary substrate.
- Fermentation - The bacterial culture ferments the sugars, producing diacetyl as a byproduct. This occurs under controlled anaerobic conditions.
- Extraction - After a specified period, diacetyl is extracted from the fermentation solution.
- Purification - The diacetyl is purified to remove impurities.
It appears as a colorless liquid.

What it is for and where
Cosmetics
Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances. Used for perfumes and aromatic raw materials.
Food
Diacetyl is a by-product of fermentation in alcoholic beverages and is added as a synthetic flavoring agent in foods to impart a buttery flavor. Naturally present in certain alcoholic beverages like beer and wine due to fermentation.
Electronic cigarettes
Diacetyl is included as a synthetic flavoring agent.
Safety
Diacetyl has exhibited toxicity in numerous studies in recent years that has concerned regulators (1), and its exposure to high vapor concentrations can cause long-term impairment in lung function (2) as well as disruption of iron homeostasis (3) and metabolic changes in protein structure (4).
Prolonged and significant exposure to diacetyl, especially in industrial contexts, has been linked to a lung disease called "bronchiolitis obliterans" or "popcorn lung". As a result, the use of diacetyl in various industrial settings has become a health concern.
- Molecular Formula C4H6O2
- Molecular Weight 86.09 g/mol
- CAS 431-03-8
- UNII K324J5K4HM
- EC Number 207-069-8
- DTXSID6021583
- Nikkaji J2.586K
- Metabolomics Workbench ID 5418
Synonyms:
- 2,3-Butanedione
- Biacetyl
- Diacetyl
- Diketobutane
- Dimethyldiketone
- Dimethylglyoxal
References_____________________________________________________________________
(1) Shibamoto T. Diacetyl: occurrence, analysis, and toxicity. J Agric Food Chem. 2014 May 7;62(18):4048-53. doi: 10.1021/jf500615u. Epub 2014 Apr 25. PMID: 24738917.
Abstract. Diacetyl possesses a butter-like flavor and has been widely used as a flavoring agent. It forms from sugars and lipids via various bacteria and heat treatment in various foods and beverages, such as milk. The toxicity of diacetyl, especially when inhaled, has recently attracted the attention not only of consumers but also of regulatory agencies. Even though accurate quantitative analysis of diacetyl is extremely important in evaluating its possible adverse effects, precise quantitative analysis of diacetyl in foods and beverages, as well as in ambient air, is considerably difficult because it is highly reactive and soluble in water. Among the many analytical methods developed for measuring diacetyl, preparation of 2,3-dimethylquinoxaline followed by gas chromatography has been most commonly used in the analysis of various foods, beverages, and air samples. This mini-review summarizes the formation mechanisms, analytical methods, occurrence, and toxicity of diacetyl.
(2) Brass DM, Palmer SM. Models of toxicity of diacetyl and alternative diones. Toxicology. 2017 Aug 1;388:15-20. doi: 10.1016/j.tox.2017.02.011.
(3) Ghio AJ, Soukup JM, Dailey LA, Roggli VL, Crumbliss AL, Palmer SM. Diacetyl exposure disrupts iron homeostasis in animals and cells. Inhal Toxicol. 2021 May-Jul;33(6-8):268-274. doi: 10.1080/08958378.2021.1989092.
Abstract. Objective: Several mechanisms have been proposed for the biological effect of diacetyl. We tested the postulate that animal and cell exposures to diacetyl are associated with a disruption in iron homeostasis....Results: After exposure of animals to diacetyl, there were airway polypoid lesions which stained positively for both iron and the intracellular storage protein ferritin. Trichrome stain showed a deposition of collagen immediately adjacent to accumulated metal following diacetyl exposure. In in vitro cell exposures, FAC increased non-heme iron concentration but co-incubations of FAC and diacetyl elevated levels to significantly greater values. Levels of ferritin were increased with exposures of BEAS-2B and THP-1 cells to FAC but were similarly greater after co-exposure with FAC and diacetyl. Conclusions: Results of animal and cell studies support a disruption of iron homeostasis by diacetyl. It is proposed that, following internalization, diacetyl complexes intracellular sources of iron. The cell recognizes a loss of its requisite iron to diacetyl and imports greater concentrations of the metal.
(4) Jedlicka LDL, Silva JDC, Balbino AM, Neto GB, Furtado DZS, da Silva HDT, Cavalcanti FBC, van der Heijden KM, Penatti CAA, Bechara EJH, Assunção NA. Effects of Diacetyl Flavoring Exposure in Mice Metabolism. Biomed Res Int. 2018 Jun 28;2018:9875319. doi: 10.1155/2018/9875319.
Abstract. Diacetyl is a flavoring that imparts a buttery flavor to foods, but the use or exposure to diacetyl has been related to some diseases. We investigated the effect of oral intake of diacetyl in male and female C57/Bl mice. We performed a target metabolomics assay using ultraperformance liquid chromatography paired with triple quadrupole mass spectrometry (UPLC-MS/MS) for the determination and quantification of plasmatic metabolites. We observed alterations in metabolites present in the urea and tricarboxylic acid (TCA) cycles. Peroxynitrite plasmatic levels were evaluated by a colorimetric method, final activity of superoxide dismutase (SOD) was evaluated by an enzymatic method, and mouse behavior was evaluated. Majority of the assay showed differences between control and treatment groups, as well as between genders. This may indicate the involvement of sex hormones in the regulation of a normal metabolic profile, and the implication of sex differences in metabolite disease response.
![]() Diacetyl
Diacetyl