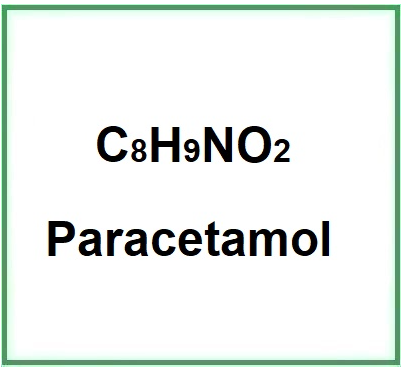
Acetaminophen or Paracetamol or N-(4-Hydroxyphenyl)acetamide, first marketed in the United States in 1950 and in U.K. in 1956, is an active ingredient,analgesic and antipyretic, used for the treatment of influenza and colds, present in the hundreds of drugs that can be sold without a prescription.
Take only under medical supervision
Paracetamol has antipyretic properties similar to aspirin, but unlike aspirin, it has no anti-inflammatory activity. The antipyretic properties of both paracetamol and aspirin are based on the inhibition of prostglandins and other substances by arachidonic acid (1).
The maximum daily dose for adults is 4 grams per day (12). It is usually considered safe even if, in overdose, it is associated with liver injury. Avoid using alcohol, smoking, or other drugs while taking this medicine.
It's also a drug for patients who suffer from pain associated with osteoarthritis.
This study found that paracetamol is effective in reducing fever and symptoms and reflects current recommendations that only treat symptoms and not routine prophylaxis (3).
In particular, in elderly people it is important to strictly follow the advice on maximum doses that should not be exceeded (4).
In infants undergoing major surgery, morphine is used as standard postoperative analgesic therapy. To reduce the amount of morphine, paracetamol has been successfully used (5).
In children the use of aspirin has almost disappeared and paracetamol has become the primary treatment for analgesia and antipyresis (6).
Contraindications
After ingestion, 90% of paracetamol is metabolized in the liver in combination with glucuronic acid, sulfuric acid and cystine (7).
Paracetamol overdose is the main causative factor of acute liver failure (8) (9) but some natural products have demonstrated hepatoprotective effects against paracetamol-induced hepatotoxicity (10).
Taking paracetamol on an occasional basis does not pose any problems, but taking it regularly and continuously for a few days can increase the risk of cardiovascular disease (11).
Like all drugs it can cause side effects. Always ask the physician.
More about paracetamol
Typical optimal characteristics of the commercial product Paracetamol
| Appearance | Powder fine white |
| Boiling Point | 387.8±25.0 °C at 760 mmHg |
| Melting Point | 168.0°C to 172.0°C |
| Density | 1.2930g/mL |
| Flash Point | 188.4±23.2 °C |
| PSA | 49.33000 |
| LogP | 0.34 |
| Index of Refraction | 1.619 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Safety |  |
- Molecular Formula: C8H9NO2
- Linear Formula: CH3CONHC6H4OH
- Molecular Weight: 151.16 g/mol
- CAS: 103-90-2
- EC Number: 203-157-5
- UNII 362O9ITL9D
- InChI=1S/C8H9NO2/c1-6(10)9-7-2-4-8(11)5-3-7/h2-5,11H,1H3,(H,9,10)
- InChl Key RZVAJINKPMORJF-UHFFFAOYSA-N
- SMILES CC(=O)NC1=CC=C(C=C1)O
- IUPAC N-(4-hydroxyphenyl)acetamide
- ChEBI 46195
- DSSTox Substance ID: DTXSID2020006
- MDL number MFCD00002328
- PubChem Substance ID 329820028
- NACRES NA.24
- Beilstein/REAXYS Number 2208089
Synonyms:
- N-(4-hydroxyphenyl)acetamide
- Panadol
- N-Acetyl-p-aminophenol
- Algotropyl
- p-Acetamidophenol
- Hydroxyacetanilide
- p-Hydroxyacetanilide
References__________________________________________________________________
(1) Meredith TJ, Goulding R. Paracetamol. Postgrad Med J. 1980;56(657):459–473. doi:10.1136/pgmj.56.657.459
(2) Food and Drug Administration, HHS. Organ-specific warnings; internal analgesic, antipyretic, and antirheumatic drug products for over-the-counter human use; final monograph. Final rule. Fed Regist. 2009 Apr 29;74(81):19385-409.
(3) Rose MA, Juergens C, Schmoele-Thoma B, Gruber WC, Baker S, Zielen S. An open-label randomized clinical trial of prophylactic paracetamol coadministered with 7-valent pneumococcal conjugate vaccine and hexavalent diphtheria toxoid, tetanus toxoid, 3-component acellular pertussis, hepatitis B, inactivated poliovirus, and Haemophilus influenzae type b vaccine. BMC Pediatr. 2013;13:98. Published 2013 Jun 21. doi:10.1186/1471-2431-13-98
(4) What dose of paracetamol for older people?. Drug Ther Bull. 2018;56(6):69–72. doi:10.1136/dtb.2018.6.0636
(5) Krekels EH, van Ham S, Allegaert K, et al. Developmental changes rather than repeated administration drive paracetamol glucuronidation in neonates and infants. Eur J Clin Pharmacol. 2015;71(9):1075–1082. doi:10.1007/s00228-015-1887-y
(6) Cranswick N, Coghlan D. Paracetamol efficacy and safety in children: the first 40 years. Am J Ther. 2000;7(2):135–141. doi:10.1097/00045391-200007020-00010
(7) Jóźwiak-Bebenista M, Nowak JZ. Paracetamol: mechanism of action, applications and safety concern. Acta Pol Pharm. 2014;71(1):11–23.
(8) Bretherick AD, Craig DG, Masterton G, et al. Acute liver failure in Scotland between 1992 and 2009; incidence, aetiology and outcome. QJM. 2011;104(11):945–956. doi:10.1093/qjmed/hcr098
(9) Minsart C, Liefferinckx C, Lemmers A, et al. New insights in acetaminophen toxicity: HMGB1 contributes by itself to amplify hepatocyte necrosis in vitro through the TLR4-TRIF-RIPK3 axis. Sci Rep. 2020;10(1):5557. Published 2020 Mar 27. (z) doi:10.1038/s41598-020-61270-1
(10) Chang L, Xu D, Zhu J, Ge G, Kong X, Zhou Y. Herbal Therapy for the Treatment of Acetaminophen-Associated Liver Injury: Recent Advances and Future Perspectives. Front Pharmacol. 2020;11:313. Published 2020 Mar 11. doi:10.3389/fphar.2020.00313
(11) Perrin G, Arnoux A, Berdot S, Katsahian S, Danchin N, Sabatier B. Association Between Exposure to Effervescent Paracetamol and Hospitalization for Acute Heart Failure: A Case-Crossover Study. J Clin Pharmacol. 2022 Jan 16. doi: 10.1002/jcph.2027.