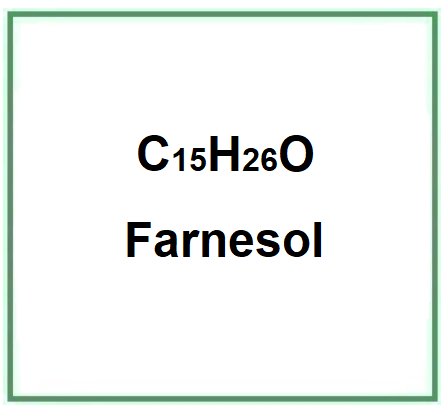
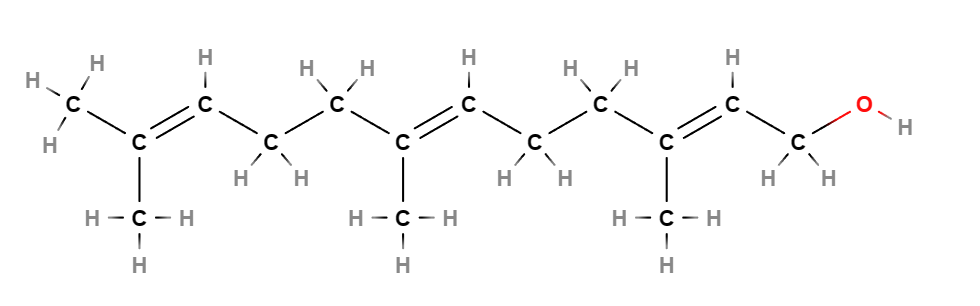
Farnesol is a natural sesquiterpenoid alcohol, a component of many essential oils such as citronella, neroli, cyclamen, lemon, and rosemary.
Description of raw materials used in production and their functions.
- Isoprene. This is a key building block for many natural terpenes, including farnesol. It is naturally found in various plants and can also be produced synthetically.
Step-by-step summary of industrial chemical synthesis process.
- Production of Isoprene. Isoprene can be produced either from natural sources, like during the distillation of natural rubber, or through synthetic methods.
- Polymerization. The isoprene undergoes polymerization to form farnesene, which is a direct precursor to farnesol.
- Hydrogenation. Farnesene is hydrogenated to produce farnesol.
- Purification. The produced farnesol is purified using various techniques like distillation to ensure a high-quality product.
It appears in the form of a white powder.

What it is for and where
Medical
Antimicrobial Agent. Farnesol has shown antimicrobial activity, which can be useful in preventing the growth of certain bacteria and fungi (1).
Dental Health. Some studies suggest that Farnesol, combined with other agents, can inhibit the formation of dental plaque (2).
Cosmetics
It is a restricted ingredient as III/82 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-
Deodorant agent. When substances that give off an unpleasant odour are included in cosmetic formulations (typical examples are methyl mercaptan and hydrogen sulphide derived from garlic), deodorants attenuate or eliminate the unpleasant exhalation. It helps counteract the formation of bad odours on body surfaces.
Perfuming. Unlike fragrance, which can also contain slightly less pleasant or characteristic odours, the term perfume indicates only very pleasant fragrances. Used for perfumes and aromatic raw materials.
Commercial Applications
Cosmetics and Personal Care. Farnesol is frequently used in deodorants for its antibacterial properties, which help inhibit the growth of odor-causing bacteria on the skin. It's also found in perfumes due to its pleasant, mild scent.
Perfumery. Farnesol is a natural component of many essential oils, including rose, orange blossom, and citronella. It's used in many perfumes as a fragrance ingredient and for its ability to blend and enhance other fragrances.
Other Applications
Research Tool. Farnesol is used in scientific research as a quorum-sensing molecule, especially in studies related to Candida albicans, a type of fungus.
Pesticides. Farnesol's antimicrobial properties have led to its exploration as a potential component in natural or less-toxic pesticides.
Safety
Farnesol is believed to have sensitization potential and requires labeling in the European Union if it is used in a consumer product (3).
- Molecular Formula C15H26O
- Molecular Weight 222.37 g/mol
- CAS 106-28-5
- UNII X23PI60R17
- EC Number 225-004-1
- DTXSID2040789
- FEMA 2478
Synonyms:
trans,trans-Farnesol
References_____________________________________________________________________
(1) Polke M, Leonhardt I, Kurzai O, Jacobsen ID. Farnesol signalling in Candida albicans - more than just communication. Crit Rev Microbiol. 2018 Mar;44(2):230-243. doi: 10.1080/1040841X.2017.1337711.
Abstract. Candida albicans is a successful colonizer of the human host, which can, under certain circumstances cause a range of clinically diverse infections. Important virulence-associated traits of the fungus, such as the dimorphic switch and biofilm formation, are controlled by the quorum sensing molecule farnesol. Given the potential of farnesol as a novel antifungal drug, there has been increasing research into the mechanism underlying farnesol sensing and action in C. albicans. However, despite the identification of various factors involved in farnesol signalling, its exact mode of action remains largely unclear. This review provides an overview of the currently known aspects of farnesol production, sensing and action within C. albicans. We also illustrate the characteristic of C. albicans to simultaneously produce and tolerate high farnesol concentrations that are lethal to other microbes. Furthermore, we summarize new literature on the role of farnesol in the interaction of C. albicans with the human host and highlight its action as a potent immunomodulatory molecule.
(2) Leyva Del Rio D, Sartori N, Tomblin NB, Phark JH, Pardi V, Murata RM, Duarte S Jr. Bioactive Dental Adhesive System With tt-Farnesol: Effects on Dental Biofilm and Bonding Properties. Front Bioeng Biotechnol. 2020 Jul 23;8:865. doi: 10.3389/fbioe.2020.00865.
(3) Gilpin S, Maibach H. Allergic contact dermatitis caused by farnesol: clinical relevance. Cutan Ocul Toxicol. 2010 Dec;29(4):278-87. doi: 10.3109/15569527.2010.511369.
Abstract. Context: The fragrance material farnesol is cited as an infrequent but important cause of allergic contact dermatitis (ACD). It is included in the fragrance mix II patch series and requires labeling in the European Union if it is used in a consumer product. Objective: To review the existing literature to determine the causative role of farnesol in clinical contact allergy. Materials and methods: Survey of the literature on farnesol studies; predictive and clinical elicitation tests in case reports, reviews, and abstracts. Results: Predictive animal studies demonstrated in most cases that farnesol was a nonsensitizer. However, 2 local lymph node assays (LLNAs) indicated strong sensitization potential. Predictive human test data indicated a low potential, if any, for sensitization in human tests with farnesol at 10% or 12%. A few clinical reports indicated low-level allergy or questionable reactions to farnesol, with 5% being the most commonly used. There were also reports in which no reactions were seen. Discussion: Predictive testing on farnesol in animals shows conflicting results depending on the study methodology used. Human predictive patch-test data also had gaps that prevented it from being definitive in pointing to a causative relationship between farnesol and contact dermatitis. The real sensitizing potential of a material can best be determined by evaluating the clinical and epidemiological data so as to help resolve the conflicting animal and human predictive test data. Conclusions: This literature and scoring exercise showed that predictive and clinical elicitation data do not document a clear causative determination that farnesol is a frequent contact allergen. Detailed clinical relevance and patient studies should clarify the clinical problem farnesol represents.
![]() Farnesol
Farnesol