![]() Granactive retexture T
Granactive retexture T
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
10 pts from Al222
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Granactive retexture T Review Consensus 10 by Al222 (20710 pt) | 2023-Oct-13 19:02 |
| Read the full Tiiip | (Send your comment) |
Granactive retexture T or Hydroxypinacolone Retinoate
is a vitamin A derivative used in skincare products for its anti-aging properties. It is known for being less irritating compared to other forms of retinoids and for its ability to stimulate collagen production and promote cell turnover without causing redness and irritation.
The name describes the structure of the molecule
- Hydroxy refers to a -OH (hydroxyl) functional group present in the molecule.
- Pinacolone is a type of ketone, which is an organic compound characterized by a carbonyl group (a carbon atom double-bonded to an oxygen atom) bonded to two alkyl groups.
- Retinoate indicates an ester of retinoic acid, which is a form of vitamin A. Esters are compounds formed from the reaction between an acid and an alcohol.
Description of raw materials used in production
- Retinoic acid - The base compound from which Hydroxypinacolone Retinoate is derived.
- Pinacolone - Used to esterify retinoic acid.
Step-by-step summary of industrial chemical synthesis process
- Preparation of Retinoic Acid. Retinoic acid, usually sourced from beta-carotene (found in carrots and other orange vegetables), undergoes isomerization.
- Synthesis of HPR. Through esterification, retinoic acid is reacted with hydroxypinacolone, creating hydroxypinacolone retinoate.
- Purification. The HPR is purified through various methods like recrystallization or chromatography to achieve the desired purity.
- Testing. Rigorous testing for purity and efficacy is performed.
- Formulation. HPR is incorporated into various cosmetic formulations.
It is often found as a light yellow to orange viscous liquid or white powder.

What it is for and where
Cosmetics
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Medical Applications
Dermatological Treatments. It might be used in treatments meant to enhance the appearance of scars, hyperpigmentation spots, and other age-related skin conditions.
Acne Products. Owing to its comedolytic properties, it's used in products aimed at treating acne. (1).
Commercial Applications
- Cosmetics and Skincare. Leveraged for its anti-aging properties, it assists in reducing wrinkles, stimulating collagen production, and renewing the skin.
- Serums and Lotions. Often featured in serums and lotions for its ability to brighten the skin and improve skin texture.
- Exfoliating Products. Acts as a chemical exfoliant to slough off dead skin cells and reveal brighter, more youthful skin.
Potential Health Concerns
- Skin Sensitivity. Some individuals might experience skin sensitivity, irritation, or allergic reactions. Patch testing before widespread use is recommended.
- Pregnancy and Breastfeeding. The use of retinoids is generally discouraged during pregnancy and breastfeeding due to potential risk, though HPR is considered a milder form.
Regulations
Different regions (like the EU, USA, or Asia) may have specific guidelines and regulations concerning the usage levels, labeling, and claims of skincare products containing retinoids, including HPR.
In the cosmetic industry, products containing HPR should adhere to cosmetic regulation and should pass safety assessments.
Storage and Stability
Stable under ordinary conditions but may degrade with exposure to light and air. Products containing HPR should be stored in air-tight, light-protective packaging.
Environmental Impact
The impact largely depends on the manufacturing processes and sourcing of raw materials, which may involve the use of solvents and create by-products.
Packaging of cosmetic products and sustainability of ingredients might also be considered in an overall environmental impact assessment.
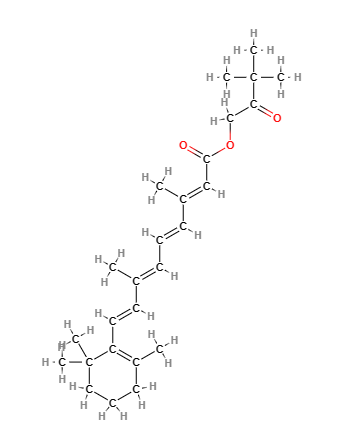 | 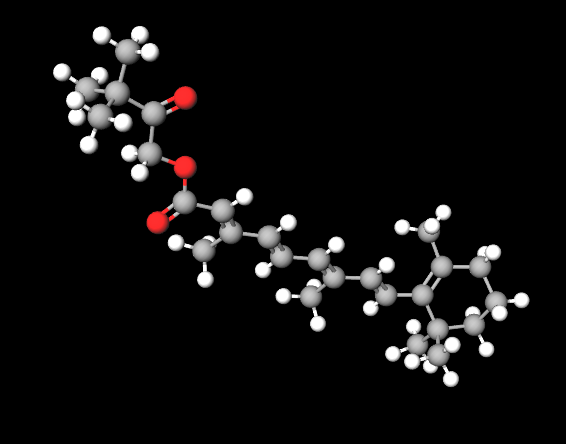 |
- Molecular Formula C26H38O3
- Molecular Weight 398.6 g/mol
- CAS 893412-73-2
- UNII NJ3V2F02E1
- EC Number 826-245-0
- Nikkaji J3.287.028G
Synonyms:
Granactive retexture T
Retinoic acid, 3,3-dimethyl-2-oxobutyl ester
References_____________________________________________________________________
(1) Villani A, Annunziata MC, Cinelli E, Donnarumma M, Milani M, Fabbrocini G. Efficacy and safety of a new topical gel formulation containing retinol encapsulated in glycospheres and hydroxypinacolone retinoate, an antimicrobial peptide, salicylic acid, glycolic acid and niacinamide for the treatment of mild acne: preliminary results of a 2-month prospective study. G Ital Dermatol Venereol. 2020 Oct;155(5):676-679. doi: 10.23736/S0392-0488.20.06581-5. Epub 2020 Sep 1. PMID: 32869963.
Abstract. Background: Acne vulgaris is a common and chronic skin disease that impacts on physical and psychological perceptions. Combination therapy with topical retinoids and antimicrobial agent is considered the preferred approach for most of the subjects affected by mild-to-moderate acne. A correct therapeutic management should include a prolonged treatment to ensure therapeutic success and to prevent recurrences. The aim of this study was to evaluate the efficacy and tolerability of a new topical gel formulation that combines retinol encapsulated in glycospheres and hydroxypinacolone retinoate, associated with an anti-microbial peptide (BIOPEP-15) salicylic acid, glycolic acid, and niacinamide as monotherapy in mild acne vulgaris....Results: Twenty-five female patients with a median age of 23.4 were enrolled. Twenty-two (88%) completed the 2-month treatment period visits. At baseline the total acne lesion number, mean (SD), was 5.5 (4) and the GAG score 9 (4). A significant (P=0.001) reduction in number of total acne lesions was observed at week 4 (-57%) and at week 8 (-80%). All patients presented a significant reduction of the GAGS score values: -42% at week 4 and -78% at week 8, confirming the clinical efficacy of the product. At baseline TEWL was 10.2 g/m2/h (1.3) and 10.7 (1.4) at week 8, thus showing that the gel did not impair the skin barrier function. Skin colorimetry was significantly (P=0.0015) reduced by the treatment in comparison with baseline (62 vs. 58). Efficacy of the gel formulation was also confirmed with RCM exams, showing a reduction of dermal inflammation and exocytosis, and an improvement of infundibular hyperkeratinization. We observed that adherence to treatment correlated positively with the improvement of the single parameters. Moreover, side effects such as erythema, dryness, and excessive xerosis were not reported, resulting in a complete adherence to the treatment. Conclusions: Our findings provide favorable evidences of the efficacy and safety of this new product as a first line treatment in patients with mild acne, or, as a maintenance therapy for prolonged periods after the suspension of a systemic treatment. Furthermore, the tolerability of this topical product and the absence of any side effects increased the adherence to the therapy.
(2) Bai D, Hu F, Xu H, Huang J, Wu C, Zhang J, Ye R. High Stability and Low Irritation of Retinol Propionate and Hydroxypinacolone Retinoate Supramolecular Nanoparticles with Effective Anti-Wrinkle Efficacy. Pharmaceutics. 2023 Feb 22;15(3):731. doi: 10.3390/pharmaceutics15030731.
Abstract. Gravi-A nanoparticles, composed of retinyl propionate (RP) and hydroxypinacolone retinoate (HPR), were prepared by encapsulating the two using the high-pressure homogenization technique. The nanoparticles are effective in anti-wrinkle treatment with high stability and low irritation. We evaluated the effect of different process parameters on nanoparticle preparation. Supramolecular technology effectively produced nanoparticles with spherical shapes with an average size of 101.1 nm. The encapsulation efficiency was in the 97.98-98.35% range. The system showed a sustained release profile for reducing the irritation caused by Gravi-A nanoparticles. Furthermore, applying lipid nanoparticle encapsulation technology improved the transdermal efficiency of the nanoparticles, thereby allowing these to penetrate deep into the dermis layer to achieve precise and sustained release of active ingredients. Gravi-A nanoparticles can be extensively and conveniently used in cosmetics and other related formulations by direct application.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2023-10-13 18:38:12 | Chemical Risk: |