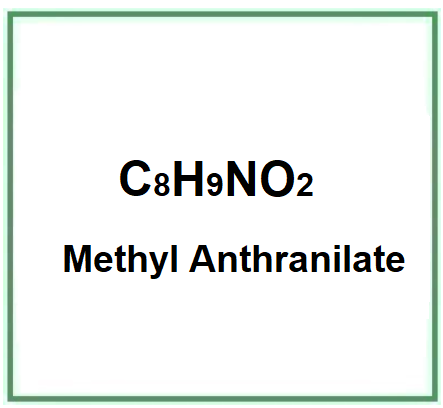
Methyl Anthranilate is an ester of anthranilic acid and has a chemical structure represented as C8H9NO2. It is often used as a flavoring in food and beverages for its grape-like scent and can also be used in perfumery and cosmetics. Additionally, methyl anthranilate has applications as a bird repellent in agriculture and other settings.
The name describes the structure of the molecule.
- Methyl- refers to a methyl group, which is a carbon atom bound to three hydrogen atoms and represented as -CH3 in chemical structures.
- Anthranilate refers to the salt or ester of anthranilic acid, an aromatic compound that contains a carboxylic acid group and an amine group.
Step-by-step Summary of Industrial Production Process.
- Raw Material Acquisition. Anthranilic acid, methanol, and other essential reagents are obtained.
- Esterification of Anthranilic Acid. Anthranilic acid is esterified with methanol, typically utilizing acid catalysts, to produce methyl anthranilate.
- Purification. The resultant methyl anthranilate is purified through various methods like distillation to achieve high purity.
- Formulation. The pure methyl anthranilate is formulated into different concentrations or combined with other substances for varied applications in food, cosmetics, and other industries.
- Quality Control. The final product undergoes testing for purity, safety, and effectiveness before being distributed.
Form and Color.
Methyl anthranilate typically appears as a colorless or slightly yellow liquid with a distinctive grape-like odor.

Commercial Applications:
Food Industry. Methyl anthranilate is used as a flavoring in various food products like beverages, candies, and chewing gums, to impart a grape flavor.
Perfumery. Used as a component in perfumes and fragranced products due to its sweet, fruity scent.
Cosmetics. It can be used in cosmetic and skincare products for its pleasant scent and to mask undesirable odors.
Bird Repellent. Methyl anthranilate is used in some bird repellents because of its ability to deter birds without harming them.
Chemical Industry. Used in the synthesis of other chemical compounds in various industrial applications.
Safety
Generally recognized as safe (1) in small amounts, but can cause skin irritation in some individuals.
- Molecular Formula C8H9NO2
- Molecular Weight 151.16 g/mol
- CAS 134-20-3
- UNII 981I0C1E5W
- EC Number 205-132-4
- DTXSID6025567
- FEMA 2682
Synonyms:
2-Aminobenzoic acid methyl ester
Methyl o-aminobenzoate
Methyl 2-aminobenzoate
References_____________________________________________________________________
(1) Api AM, Belsito D, Botelho D, Browne D, Bruze M, Burton GA Jr, Buschmann J, Dagli ML, Date M, Dekant W, Deodhar C, Fryer AD, Joshi K, La Cava S, Lapczynski A, Liebler DC, O'Brien D, Parakhia R, Patel A, Penning TM, Ritacco G, Romine J, Salvito D, Schultz TW, Sipes IG, Thakkar Y, Tokura Y, Tsang S, Wahler J. RIFM fragrance ingredient safety assessment, methyl anthranilate, CAS Registry Number 134-20-3. Food Chem Toxicol. 2017 Dec;110 Suppl 1:S290-S298. doi: 10.1016/j.fct.2017.06.003. Epub 2017 Jun 6. PMID: 28595957.
![]() Methyl Anthranilate
Methyl Anthranilate