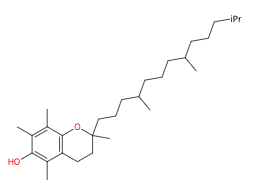
All-rac-alpha-Tocopherol is a synthetic form of vitamin E. It is often used in cosmetic products and skincare for its antioxidant and protective properties.
The name describes the structure of the molecule:
- all-rac indicates that the molecule is a racemic mixture, meaning it contains equal amounts of both enantiomers (mirror forms) of a chiral molecule.
- alpha indicates the position and orientation of functional groups in the tocopherol molecule. There are different forms of tocopherol (alpha, beta, gamma, delta), and "alpha" has the highest vitamin E activity.
- Tocopherol is the chemical name for vitamin E, a natural antioxidant that protects cells from free radical damage.
Raw Materials Used in Production.
All-rac-alpha-tocopherol is a synthetic form of vitamin E. The raw materials used for its synthesis include isophorone and trimethylhydroquinone.
Step-by-step Summary of Industrial Production Process.
- Production of intermediates. Starting from raw materials like isophorone, chemical intermediates such as trimethylhydroquinone are produced.
- Condensation. The trimethylhydroquinone is then condensed with isophorone to form a tocopherol intermediate.
- Hydrogenation. This intermediate undergoes a hydrogenation process to yield all-rac-alpha-tocopherol.
- Purification. The synthetic form of vitamin E is purified to remove any impurities through processes like distillation and crystallization.
Form and Color.
All-rac-alpha-tocopherol is generally a viscous, colorless to pale yellow liquid.

Alpha tocopherol is one of the four isomer components that constitute tocopherol: alpha-tocopherol, beta-tocopherol, delta-tocopherol and/or gamma-tocopherol.
Alpha tocopherol occurs naturally in cereals, in oils and, in particular in:
It is a fundamental antioxidant and can be created using a chemical process.
Its antioxidant action has effects on the brain (1), when, in the cardiovascular system, there are problems of diabetes caused by LDL cholesterol (2) and in liver diseases (3)
In the medical field it is an adjuvant to treat vascular diseases, as prevention for cellular diseases and for a correct functioning of the immune system.
No positive action, however, was found towards prostate cancer, as demonstrated by this study of very long duration on many samples (4).
What it is used for and where
Medical
Its antioxidant action has effects on the brain (1), when, in the cardiovascular system, there are problems of diabetes caused by LDL cholesterol (2) and in liver diseases (3)
In the medical field it is an adjuvant to treat vascular diseases, as prevention for cellular diseases and for a correct functioning of the immune system.
No positive action, however, was found towards prostate cancer, as demonstrated by this study of very long duration on many samples (4).
Food
Ingredient included in the list of European food additives as E307, antioxidant.
Cosmetics
Antioxidant agent. Ingredient that counteracts oxidative stress and prevents cell damage. Free radicals, pathological inflammatory processes, reactive nitrogen species and reactive oxygen species are responsible for the ageing process and many diseases caused by oxidation.
Fragrance. It plays a decisive and important role in the formulation of cosmetic products as it provides the possibility of enhancing, masking or adding fragrance to the final product, increasing its marketability. The consumer always expects to find a pleasant or distinctive scent in a cosmetic product.
Skin conditioning agent - Miscellaneous. This ingredient has the task of modifying the condition of the skin when it is damaged or dry by reducing its flakiness and restoring its elasticity.
Skin conditioning agent - Occlusive. This ingredient has the task of modifying the condition of the skin when it is damaged or dry by reducing flaking and restoring elasticity. It has a strong lipophilic character and is identified as an occlusive ingredient; it is generally composed of oily and fatty materials that remain on the skin surface and reduce trans epidermal water loss.
"Alpha-tocopherol studies"
"Alpha tocopherol vitamin E studies"
- Molecular Formula: C29H50O2
- Molecular Weight: 430.717 g/mol
- CAS: 59-02-9
- EC Number: 200-201-5 200-412-2
- UNII: N9PR3490H9
- PubChem Substance ID 24899979
- MDL number MFCD00072045
- Beilstein Registry Number 4712525
- DSSTox ID DTXSID0026339
- IUPAC (2R)-2,5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydrochromen-6-ol
- InChl=1S/C29H50O2/c1-20(2)12-9-13-21(3)14-10-15-22(4)16-11-18-29(8)19-17-26-25(7)27(30)23(5)24(6)28(26)31-29/h20-22,30H,9-19H2,1-8H3/t21-,22-,29-/m1/s1
- InChl Key GVJHHUAWPYXKBD-IEOSBIPESA-N
- SMILES CC1=C(C2=C(CCC(O2)(C)CCCC(C)CCCC(C)CCCC(C)C)C(=C1O)C)C
- ChEBI 18145
- NACRES NA.75
- eCl@ss 34058016
- Nikkaji J24.260H
- NCI C68313 C2832 C74960
- RXCUI 237099 1236136 11256
- Metabolomics Workbench 29096
- Pharos Ligand 85BJCGZ83ZT5
References___________________________________________________________________________
(1) Sakr HF, Abbas AM, El Samanoudy AZ. Effect of vitamin E on cerebral cortical oxidative stress and brain-derived neurotrophic factor gene expression induced by hypoxia and exercise in rats. J Physiol Pharmacol. 2015 Apr;66(2):191-202.
(2) Shirpoor A, Norouzi L, Nemati S, Khadem Ansari MH. Protective Effect of Vitamin E against Diabetes-Induced Oxidized LDL and Aorta Cell Wall Proliferation in Rat. Iran Biomed J. 2015 Apr;19(2):117-23.
(3) Miyanishi K, Hoki T, Tanaka S, Kato J. Prevention of hepatocellular carcinoma: Focusing on antioxidant therapy. World J Hepatol. 2015 Mar 27;7(3):593-9. doi: 10.4254/wjh.v7.i3.593. Review.
Khastar H. Protective effects of vitamin E against liver damage caused by renal ischemia reperfusion. Ren Fail. 2015 Feb 2:1-3.
(4) Ramamoorthy V, Rubens M, Saxena A, Shehadeh N. Selenium and Vitamin E for Prostate Cancer - Justifications for the SELECT Study. Asian Pac J Cancer Prev. 2015;16(7):2619-27.
![]() all-rac-alpha-Tocopherol
all-rac-alpha-Tocopherol