![]() Chlormequat chloride
Chlormequat chloride
Rating : 5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
0 pts from Al222
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Chlormequat chloride by Al222 (20724 pt) | 2024-Feb-20 10:20 |
| Read the full Tiiip | (Send your comment) |
Chlormequat Chloride is a plant growth regulator extensively utilized in agriculture to encourage denser plant growth and enhance resistance against lodging. Known scientifically as chlormequat chloride, it falls under the category of growth retardants, a group of chemicals designed to control and modulate plant growth. Its application spans a variety of crops, including cereals, vegetables, and ornamental plants, aiming to manage plant height effectively and facilitate easier harvesting processes.
Composition
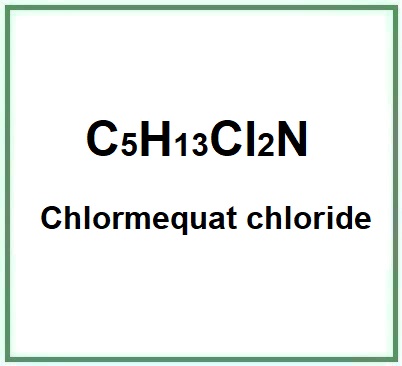
Chlormequat chloride, with the chemical formula C5H13Cl2N, operates by inhibiting gibberellin biosynthesis. Gibberellins are plant hormones that play a crucial role in regulating plant growth and development. By limiting the production of gibberellins, chlormequat chloride effectively reduces cell elongation, leading to sturdier and more compact plant structures.
Agronomic Properties
The primary function of chlormequat chloride is to prevent excessive stem growth, thereby reducing the risk of plant lodging. Lodging is a condition where plants fall over due to weak stems, significantly affecting yield and quality. By ensuring a more robust plant architecture, chlormequat chloride helps in maintaining optimal crop health and productivity.
Use in Agriculture
Chlormequat chloride is applied through foliar sprays or soil treatments at specific stages of plant growth. The timing and method of application are critical to achieving the desired growth regulation effects. It is particularly beneficial in intensive farming practices where optimal plant density and structure are crucial for mechanical harvesting.
Safety
The use of chlormequat chloride is regulated by agricultural and environmental authorities worldwide (1). Guidelines specify the maximum residue limits (MRLs) in food products and outline safe application practices to minimize potential risks to human health (2) and the environment. Adherence to these guidelines ensures that the benefits of chlormequat chloride in agriculture do not come at the expense of safety.
Environmental Concerns
Environmental stewardship is essential when using chlormequat chloride. Measures should be taken to prevent runoff into water bodies and minimize exposure to non-target organisms. The chemical's impact on the environment depends on various factors, including application rates, local climate conditions, and soil characteristics.
Benefits and Limitations
Chlormequat chloride offers significant advantages in crop management, particularly in enhancing yield and quality by optimizing plant growth. However, its effectiveness can vary based on environmental conditions, crop species, and specific agricultural practices. Continuous research and field trials help in understanding its broader implications and in developing integrated crop management strategies that include the use of growth regulators like chlormequat chloride.
This comprehensive overview of chlormequat chloride underscores its importance in modern agriculture, highlighting the need for responsible use and adherence to regulatory standards to harness its benefits while safeguarding human health and the environment.
Chemical Industrial Synthesis Process
The chemical synthesis of Chlormequat can be achieved through the reaction of choline at 200 °C in the presence of chloride ions, likely via a nucleophilic substitution mechanism. This alternative synthesis route provides a method for producing Chlormequat chloride, leveraging the availability of choline as a starting material. Here's a more detailed look at this synthesis process:
- Reaction Setup. The process begins with the preparation of a reaction mixture containing choline, a naturally occurring quaternary ammonium compound. Chloride ions are introduced into the mixture, which can be sourced from hydrochloric acid or other chloride salts.
- Heating. The reaction mixture is heated to 200 °C. At this elevated temperature, the choline undergoes nucleophilic substitution with the chloride ions. The high temperature is crucial for facilitating the reaction kinetics and achieving a significant conversion rate.
- Nucleophilic Substitution. The mechanism likely involves the displacement of a leaving group from the choline by chloride ions. Given choline's structure, the reaction may proceed through the formation of an intermediate that ultimately leads to the generation of Chlormequat chloride.
- Cooling and Isolation. After the reaction has proceeded to completion, the mixture is cooled to room temperature. The product, Chlormequat chloride, may then be isolated from the reaction mixture through techniques such as precipitation, filtration, or centrifugation.
- Purification. The isolated Chlormequat chloride is purified to remove any unreacted choline, by-products, or other impurities. Purification steps can include recrystallization, washing with solvents, or chromatographic methods to ensure high purity of the final product.
- Quality Control. The synthesized Chlormequat chloride undergoes rigorous quality control testing. Analytical methods such as NMR (Nuclear Magnetic Resonance), HPLC (High-Performance Liquid Chromatography), and mass spectrometry are employed to confirm the chemical structure, purity, and concentration of the product.
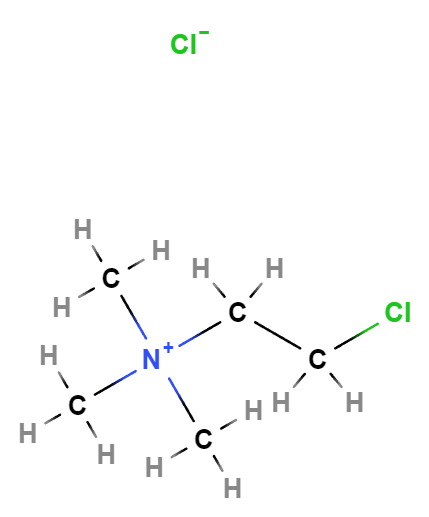 | 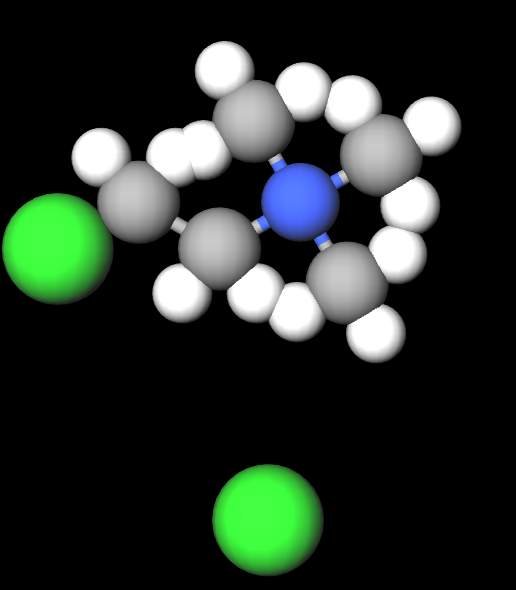 |
Molecular Formula C5H13Cl2N
Molecular Weight
CAS 999-81-5
UNII PPL2215L82
EC Number 213-666-4
DTXSID6020303
Synonyms
- Chlorinecolinchloride
- Chlorocholine Chloride
Bibliografia_____________________________________________________________________
(1) Guo XL, Jia CH, Zhao EC, Xu YJ, Han LJ, Jiang SR. Dissipation and residues of chlormequat in wheat and soil. Bull Environ Contam Toxicol. 2010 Feb;84(2):221-4. doi: 10.1007/s00128-009-9920-4. Epub 2009 Dec 4. PMID: 19960179.
Abstract. A specific, sensitive method was developed for the analysis of chlormequat in wheat and soil by high performance chromatography/mass spectrometry. The fortified recoveries of soil were from 75.08% to 96.55%, with RSD 3.34%-15.18%, the limit of detection of the analytical method was 0.05 ng at a signal-to-noise ratio of 3, and the limit of quantification was 0.05, 0.1, 0.5 mg/kg for soil, wheat plants and wheat grain, respectively. The degradation dynamics and final residues of chlormequat in Beijing and Changchun were investigated. The half-life of chlormequat in wheat plants were 3.15 days in Beijing and 4.56 days in Changchun, while the half-life in soil was 3.88 days in Beijing and 4.51 days in Changchun. The final residues of chlormequat in soil were not detectable, and the final residues of chlormequat in wheat grain were below 0.50 mg/kg except for 3.51 mg/kg from high dosage plot of Changchun. The fact that all the final residues were below 5 mg/kg (GB2763 in National standards of the People's Republic of China, maximum residue limits for pesticide in food, Beijing, 2005) suggested that chlormequat could be safely used in wheat crops with the suitable dosage and application.
(2) Galea KS, MacCalman L, Jones K, Cocker J, Teedon P, Cherrie JW, van Tongeren M. Urinary biomarker concentrations of captan, chlormequat, chlorpyrifos and cypermethrin in UK adults and children living near agricultural land. J Expo Sci Environ Epidemiol. 2015 Nov-Dec;25(6):623-31. doi: 10.1038/jes.2015.54.
Abstract. There is limited information on the exposure to pesticides experienced by UK residents living near agricultural land. This study aimed to investigate their pesticide exposure in relation to spray events. Farmers treating crops with captan, chlormequat, chlorpyrifos or cypermethrin provided spray event information. Adults and children residing ≤100 m from sprayed fields provided first-morning void urine samples during and outwith the spray season. Selected samples (1-2 days after a spray event and at other times (background samples)) were analysed and creatinine adjusted. Generalised Linear Mixed Models were used to investigate if urinary biomarkers of these pesticides were elevated after spray events. The final data set for statistical analysis contained 1518 urine samples from 140 participants, consisting of 523 spray event and 995 background samples which were analysed for pesticide urinary biomarkers. For captan and cypermethrin, the proportion of values below the limit of detection was greater than 80%, with no difference between spray event and background samples. For chlormequat and chlorpyrifos, the geometric mean urinary biomarker concentrations following spray events were 15.4 μg/g creatinine and 2.5 μg/g creatinine, respectively, compared with 16.5 μg/g creatinine and 3.0 μg/g creatinine for background samples within the spraying season. Outwith the spraying season, concentrations for chlorpyrifos were the same as those within spraying season backgrounds, but for chlormequat, lower concentrations were observed outwith the spraying season (12.3 μg/g creatinine). Overall, we observed no evidence indicative of additional urinary pesticide biomarker excretion as a result of spray events, suggesting that sources other than local spraying are responsible for the relatively low urinary pesticide biomarkers detected in the study population.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2024-02-20 09:42:56 | Chemical Risk: |