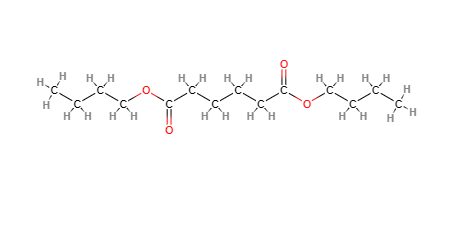
Dibutyl Adipate is an ester obtained from the reaction between adipic acid and butanol. Esters are organic compounds that form when a carboxylic acid group of an acid reacts with the hydroxyl group of an alcohol, eliminating a molecule of water. In the case of Dibutyl Adipate, two molecules of butanol react with one molecule of adipic acid, forming the ester.
Chemical Industrial Synthesis Process
- The production of Dibutyl Adipate, an adipic acid ester used as a plasticizer in polymers and as an ingredient in cosmetics for its emollient properties, follows an esterification process. Here is a detailed overview of the process.
- Preparation. Adipic acid and butyl alcohol (butanol) are prepared as reagents for the esterification reaction. The purity of these reagents is crucial for the quality of the final product.
- Esterification. Adipic acid reacts with butyl alcohol in the presence of an acid catalyst, such as sulfuric acid, to form Dibutyl Adipate and water as a by-product. The reaction is typically conducted by heating the reactive mixture at controlled temperatures.
- Water Removal. Water produced as a by-product of the reaction is removed to shift the chemical equilibrium towards product formation and increase yield. This can be done using techniques such as azeotropic distillation.
- Purification. The crude Dibutyl Adipate is purified to remove impurities, reagent residues, and by-products. Purification can include vacuum distillation, which helps to achieve a high-purity product.
- Quality Control. The purified Dibutyl Adipate undergoes quality control checks to verify its purity, chemical composition, and physical properties. These tests can include gas chromatography (GC), infrared spectroscopy (IR), and molecular weight determination.
Appearance
Typically appears as an oily, transparent, and colorless liquid with a slight characteristic odor.
What it is for and where
It is used in numerous cosmetic, pharmaceutical and skincare products for its ability to improve the texture, stability, and consistency of formulations, as well as imparting a soft and smooth feeling on the skin.
Cosmetics - INCI Functions
Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
Film-forming agent. It produces, upon application, a very thin continuous film with an optimal balance of cohesion, adhesion and stickiness on skin, hair or nails to counteract or limit damage from external phenomena such as chemicals, UV rays and pollution.
Plasticiser. Ingredient added to the formulation with the purpose of retaining fragrance and colour, increasing flexibility, flowability, deformability, durability of various ingredients allowing better processing. It softens and makes flexible synthetic polymers that otherwise could not be easily processed, stretched or deformed.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Solvent. It is the substance for dissolving or dispersing surfactants, oils, dyes, flavourings, bactericidal preservatives in solution.In fact, it dissolves other components present in a cosmetic formulation. Solvents are generally liquid (aqueous and non-aqueous).
Molecular Formula C14H26O4
Molecular Weight
CAS 105-99-7
UNII F4K100DXP3
EC Number 203-350-4
DTXSID2021866
Synonyms
- Dibutyl hexanedioate
- Butyl adipate
- Di-n-butyl adipate
Bibliografia_____________________________________________________________________
(1) Andersen A. Amended final report of the safety assessment of dibutyl adipate as used in cosmetics. Int J Toxicol. 2006;25 Suppl 1:129-34. doi: 10.1080/10915810600716679. PMID: 16835133.
Abstract. Dibutyl Adipate, the diester of butyl alcohol and adipic acid, functions as a plasticizer, skin-conditioning agent, and solvent in cosmetic formulations. It is reportedly used at a concentration of 5% in nail polish and 8% in suntan gels, creams, and liquids. Dibutyl Adipate is soluble in organic solvents, but practically insoluble in water. Dibutyl Adipate does not absorb radiation in the ultraviolet (UV) region of the spectrum. Dibutyl Adipate is not toxic in acute oral or dermal animal toxicity tests. In a subchronic dermal toxicity study, 1.0 ml/kg day-1 caused a significant reduction in body weight gain in rabbits, but 0.5 ml/kg/day1 was without effect. In a study with dogs, no adverse effects were observed when an emulsion containing 6.25% Dibutyl Adipate was applied to the entire body twice a week for 3 months. Dibutyl Adipate was tested for dermal irritation using rabbits and mice and a none to minimal irritation was observed. Dibutyl Adipate at a concentration of 25% was not a sensitizer in a guinea pig maximization study. Undiluted Dibutyl Adipate was minimally irritating to the eyes of rabbits and 0.1% was nonirritating. A significant increase in fetal gross abnormalities was observed in rats given intraperitoneal injections of Dibutyl Adipate at 1.75 ml/kg on 3 separate days during gestation, but no effect was seen in animals given 1.05 ml/kg. Dibutyl Adipate was not genotoxic in either bacterial or mammalian test systems. Clinical patch tests confirmed the absence of skin irritation found in animal tests. Clinical phototoxicity tests were negative. Dibutyl Adipate at 0.1% was not an ocular irritant in two male volunteers. In a clinical test of comedogenicity, Dibutyl Adipate produced no effect. The Cosmetic Ingredient Review (CIR) Expert Panel recognized that use of Dibutyl Adipate in suntan cosmetic products will result in repeated, frequent exposure in a leave-on product. The available data demonstrate no skin sensitization or cumulative skin irritation, no comedogenicity, and no genotoxicity. Combined with the data demonstrating little acute toxicity, no skin or ocular irritation, and no reproductive or developmental toxicity, these data form an adequate basis for reaching a conclusion that Dibutyl Adipate is safe as a cosmetic ingredient in the practices of use and concentrations as reflected in this safety assessment.
![]() Dibutyl Adipate
Dibutyl Adipate