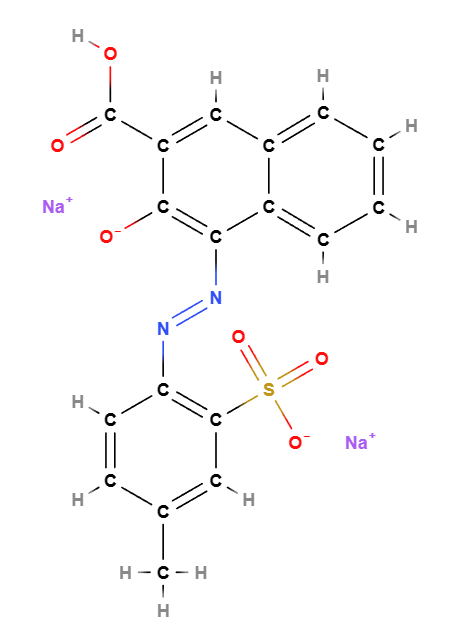
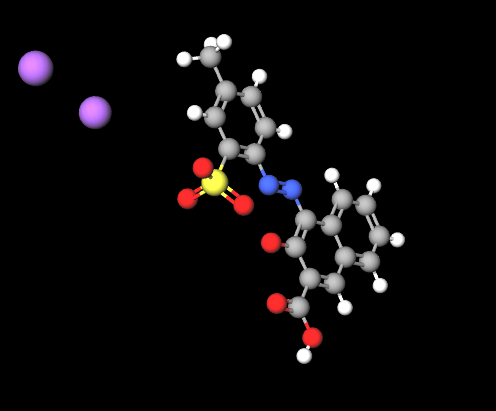
D&C Red No. 6 or Pigment Red 57 is a chemical compound, an azo synthetic red dye also known by the name CI 15850, Red 6.
The name describes the structure of the molecule:
- Pigment refers to materials that impart color to objects by absorbing light. Pigments are usually insoluble and stable in solid form, used to color paints, inks, cosmetics, and other materials.
- Red refers to the color of the pigment, indicating that this particular pigment is of a red hue.
- 57 serves as a specific identifier distinguishing this particular red pigment from others in the red pigment category. It specifies exactly which pigment is being used.
Chemical Industrial Synthesis Process
- Selection of base materials. The process begins with the selection of 3-hydroxy-2-naphthoic acid and aniline, which are the main chemical precursors.
- Condensation. The acid and aniline are fused together in the presence of an acid catalyst to form a diazonium salt.
- Coupling. The diazonium salt is then reacted with a coupling compound such as 2-naphthol, forming the crude pigment.
- Precipitation and filtration. The pigment is precipitated with water to remove excess salt and other impurities, followed by filtration to isolate the pigment.
- Drying. The filtered pigment is dried to further reduce moisture content.
- Laking. To stabilize the color and enhance resistance to light and chemicals, the pigment may be treated with substrates like barium or calcium salts.
- Quality control. Each batch of Pigment Red 57 undergoes quality testing to ensure compliance with required specifications.
It is a restricted ingredient as IV/27 (CI 15850) III/296 (Pigment Red 57) a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: Disodium 3-hydroxy-4-[(4-methyl-2-sulphonatophenyl)azo]-2-naphthoate and its insoluble barium, strontium and zirconium lakes, salts and pigments
Safety
The problem with azo dyes (monoazo or diazo) is photocatalytic degradation (1) leading to oxidation and the subsequent formation of impurities such as aromatic amines (2), some of which have carcinogenic activity.
OPINION OF THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD
PRODUCTS INTENDED FOR CONSUMERS.
CONCERNING
PIGMENT RED 57
Adopted by the SCCNFP during the 28th plenary meeting of 25 May 2004
"Pigment Red 57 (Lithol Rubine B and BK) is of low acute toxicity in rats and dogs. Repeated
and chronic oral administration to rodents up to 2 years, showed the kidney as a primary target
organ in the high doses. The lowest NOAEL (150 mg/kg/bw/day) was obtained in the 2 years
toxicity study in rats performed with the F1-generation of parent-animals pretreated at the same
dose levels. The SCF also used this study to derive an ADI for this compound.
There is no indication for corrosivity or comedogenic effects on the skin. Also no irritation on
mucous membranes was noted. From the results of the local lymph node assay it is concluded
that Pigment Red 57 is not a skin-sensitiser.
There was no convincing evidence of reproductive effects in rats and rabbits treated orally.
Pigment Red 57 (Lithol Rubine B) did not cause skin irritation in rabbits but induced skin
sensitisation in man. Diluted Lithol Rubine BK produced little irritation in man and rabbits and
failed to induce sensitisation in man.
Pigment Red 57 has been tested for the induction of gene mutations in bacterial cells and in the
mammalian cells and found negative. The in vivo study for the induction of the micronuclei is
inadequate for the evaluation.
None of the available carcinogenicity studies are reported in sufficient details to allow an
evaluation of the potential carcinogenicity of Pigment Red 57."
Chemical name: disodium;2-[(3-carboxy-2-oxidonaphthalen-1-yl)diazenyl]-5-methylbenzenesulfonate
Molecular Formula C18H12N2Na2O6S
Molecular Weight 430.3 g/mol
CAS 5858-81-1
UNII 481744AI4O
EC Number 227-497-9
DTXSID6064038
Synonyms:
D&C Red No. 6
Lithol Rubine B
Pigment Red 57
D&C Red No. 7
Lithol Rubine B Ca
Bibliografia_____________________________________________________________________
(1) Li M, He W, Liu Y, Wu H, Wamer WG, Lo YM, Yin JJ. FD&C Yellow No. 5 (tartrazine) degradation via reactive oxygen species triggered by TiO2 and Au/TiO2 nanoparticles exposed to simulated sunlight. J Agric Food Chem. 2014 Dec 10;62(49):12052-60. doi: 10.1021/jf5045052. Epub 2014 Nov 24. PMID: 25393426.
Abstract. When exposed to light, TiO2 nanoparticles (NPs) become photoactivated and create electron/hole pairs as well as reactive oxygen species (ROS). We examined the ROS production and degradation of a widely used azo dye, FD&C Yellow No. 5 (tartrazine), triggered by photoactivated TiO2 NPs. Degradation was found to follow pseudo-first order reaction kinetics where the rate constant increased with TiO2 NP concentration. Depositing Au on the surface of TiO2 largely enhanced electron transfer and ROS generation, which consequently accelerated dye degradation. Alkaline conditions promoted ROS generation and dye degradation. Results from electron spin resonance spin-trap spectroscopy suggested that at pH 7.4, both hydroxyl radical (•OH) and singlet oxygen ((1)O2) were responsible for dye discoloration, whereas at pH 5, the consumption of (1)O2 became dominant. Implications for dye degradation in foods and other consumer products that contain both TiO2 and FD&C Yellow No. 5 as ingredients are discussed.
(2) Belai N, White SR. Determination of Unsulfonated Aromatic Amines in FD&C Yellow No. 5 and FD&C Yellow No. 6 by Liquid Chromatography-Triple Quadrupole Mass Spectrometry. J AOAC Int. 2019 Mar 1;102(2):580-589. doi: 10.5740/jaoacint.18-0165.
Abstract. Background: This paper describes a simple and sensitive ultra-HPLC-triple quadrupole MS (LC-MS/MS) method for the determination of six unsulfonated aromatic amines in the color additives FD&C Yellow No. 5 (Y5) and FD&C Yellow No. 6 (Y6). The six amines determined by this method are aniline (ANL), benzidine (BNZ), 4-aminobiphenyl (4ABP), 4-aminoazobenzene (4AAB), 2-aminobiphenyl (2ABP), and 4-aminobenzonitrile (4ABN). Objective: This method is intended for use in batch certification of the color additives by the U.S. Food and Drug Administration (FDA) to ensure that each lot meets published specifications for coloring foods, drugs, and cosmetics. Methods: A modified quick, easy, cheap, effective, rugged, and safe (QuEChERS) procedure is used for extraction of the amines. Quantitative determination was performed in electrospray positive ionization and multiple-reaction monitoring modes. Results: Validation of the method demonstrated overall recovery of 101-115% and precision of 1.74-9.78% for all analytes. Excellent regression coefficients were obtained, with values >0.999. Conclusions: The validated method was successfully used for the analyses of 30 Y5 and Y6 samples and provided results that are consistent with results from the current method used by FDA, with greater sensitivity and low matrix effects. Highlights: The validation results demonstrate that the new LC-MS/MS method is applicable for use in routine batch certification.
![]() D&C Red No. 6
D&C Red No. 6