Polyhydroxystearic Acid is an organic compound derived from stearic acid, modified to include multiple hydroxyl groups. This modification increases its hydrophilicity, making it more compatible with water-based formulations. It's widely used in cosmetic and skincare products for its ability to improve the compatibility of natural oils with mineral filters and pigments. This makes it particularly effective in sunscreens and color cosmetics, where it helps to stabilize and disperse physical UV filters, such as titanium dioxide and zinc oxide, in the product formulation. Additionally, it enhances the spreadability of creams and lotions, providing a smoother application and feel on the skin.
The name describes the structure of the molecule:
- Polyhydroxy indicates the presence of multiple hydroxyl (OH) groups in the molecule's structure. These groups give the compound hydrophilic properties, enhancing its water solubility or affinity.
- stearic refers to stearic acid, a long-chain saturated fatty acid commonly found in animal and vegetable fats. In material chemistry, stearic acid is valued for its properties as a stabilizing agent and emollient.
- Acid refers to compounds in chemistry that can donate protons (H+ ions) or accept an electron pair to form bonds.
Chemical Industrial Synthesis Process
- Selection of base materials. The process begins with the selection of stearic acid, a saturated fatty acid that serves as the starting material.
- Hydrogenation. Stearic acid is treated with hydrogen in the presence of a metallic catalyst such as palladium on carbon to reduce any unsaturated double bonds and increase its reactivity.
- Oxidation. The hydrogenated stearic acid is then subjected to an oxidation process to introduce hydroxyl (-OH) groups onto the carbon chain. This step employs oxidants such as hydrogen peroxide or oxygen in the presence of a catalyst.
- Polymerization. The hydroxylated monomers are then polymerized through condensation reactions to form longer chains of polyhydroxystearic acid. This stage may involve acid or base catalysts.
- Purification. The polymeric product is purified to remove impurities and unreacted monomers, typically using techniques like precipitation or filtration.
- Quality control. Finally, the polyhydroxystearic acid undergoes quality checks to verify its purity, molecular composition, and physicochemical properties.
What it is used for and where
Cosmetics - INCI Functions
Surfactant - Emulsifying agent. Emulsions are thermodynamically unstable and are used to soothe or soften the skin and emulsify, so they need a specific, stabilising ingredient. This ingredient forms a film, lowers the surface tension and makes two immiscible liquids miscible. A very important factor affecting the stability of the emulsion is the amount of the emulsifying agent. Emulsifiers have the property of reducing the oil/water or water/oil interfacial tension, improving the stability of the emulsion and also directly influencing the stability, sensory properties and surface tension of sunscreens by modulating the filmometric performance.
Main uses and benefits of polyhydroxystearic acid.
- Emulsion Stabilizer. It helps stabilize and keep emulsions homogeneous, preventing the separation of components in products such as creams and lotions.
- Dispersion of Pigments and Sunscreens. It is particularly effective in the suspension and dispersion of pigments and nanoparticles (1), such as those used in sunscreens, enhancing their effectiveness and uniformity on the skin.
- Skin Compatibility. Thanks to its chemical structure, it improves the compatibility of cosmetic products with different skin types, making formulations more suitable for a broad spectrum of consumers.
- Product Texture Improvement. It contributes to a smoother and more pleasing texture of skincare and makeup products.
- Barrier Properties. It helps form a barrier on the skin that can protect against moisture and other external environmental factors.
- Increased Skin Hydration. It can help retain moisture in the skin, contributing to improved hydration and reducing dryness.
Safety
The Expert Panel on the Safety of Cosmetic Ingredients (Panel) has reviewed its safety. In 2019, the Panel issued a final report on the safety of polyhydroxystearic acid concluding that this ingredient is safe in current use and concentration practices as described in the safety assessment when formulated to be non-irritant (1).
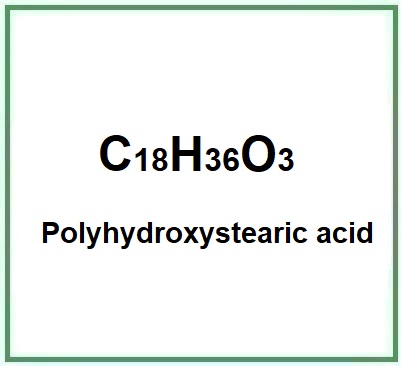
Molecular Formula C18H36O3
Molecular Weight 300.5 g/mol
CAS 27924-99-8 58128-22-6
EC number 203-366-1
UNII 933ANU3H2S
DTXSID8026725
References_____________________________________________________________________
(1) Palangetic L, Feldman K, Schaller R, Kalt R, Caseri WR, Vermant J. From near hard spheres to colloidal surfboards. Faraday Discuss. 2016 Oct 6;191:325-349. doi: 10.1039/c6fd00052e.
Abstract. This work revisits the synthesis of the colloidal particles most commonly used for making model near hard suspensions or as building blocks of model colloidal gels, i.e. sterically stabilised poly(methyl methacrylate) (PMMA) particles. The synthesis of these particles is notoriously hard to control and generally the problems are ascribed to the difficulty in synthesising the graft stabiliser (PMMA-g-PHSA). In the present work, it is shown that for improving the reliability of the synthesis as a whole, control over the polycondensation of the 12-polyhydroxystearic acid is the key. By changing the catalyst and performing the polycondensation in the melt, the chain length of the 12-polyhydroxystearic acid is better controlled, as confirmed by 1H-NMR spectroscopy. Control over the graft copolymer now enables us to make small variations of near hard sphere colloids, for example spherical PMMA particles with essentially the same core size and different stabilising layer thicknesses can now be readily produced, imparting controlled particle softness. The PMMA spheres can be further employed to create, in gram scale quantities, colloidal building blocks having geometrical and/or chemical anisotropy by using a range of mechanical deformation methods. The versatility of the latter methods is demonstrated for polystyrene latex particles as well.
(2) Shank, R. C., Slaga, T. J., Snyder, P. W., & Tilton, S. C. Safety Assessment of Polyhydroxystearic Acid, Poly (3-Hydroxyoctanoic Acid, and Polylactic Acid as Used in Cosmetics.
Slaga, T. J., Snyder, P. W., & Tilton, S. C. (2022). Safety Assessment of Polyhydroxystearic Acid, Poly (3-Hydroxyoctanoic Acid), and Polylactic Acid as Used in Cosmetics.
![]() Polyhydroxystearic Acid
Polyhydroxystearic Acid