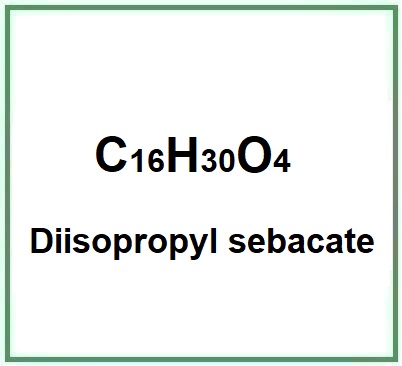
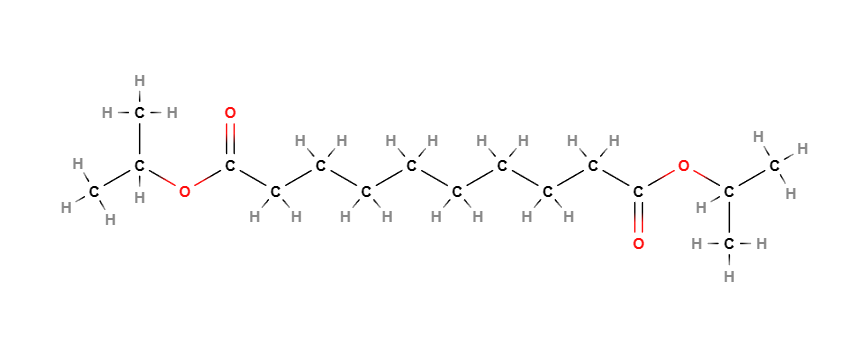
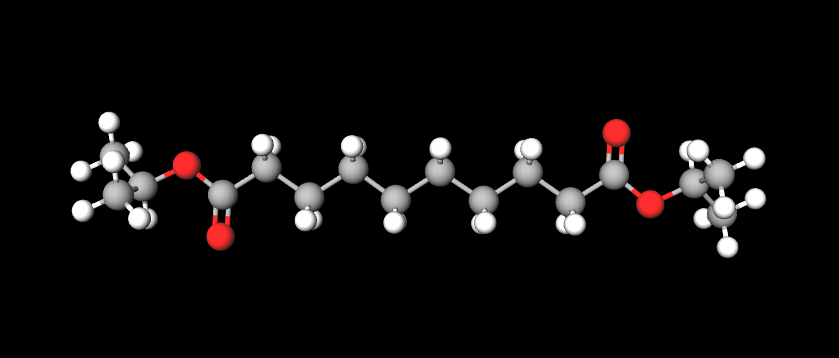
Diisopropyl Sebacate is a chemical compound, diester of isopropyl alcohol and Sebacic Acid.. It is used as an emollient and solvent in cosmetic products.
Chemical Industrial Synthesis Process
- Preparation of reagents. Sebacic acid is obtained from castor oil through a fermentation process, while isopropyl alcohol is produced by reducing acetone.
- Esterification reaction. Sebacic acid and isopropyl alcohol are combined in a reactor in the presence of an acid catalyst like sulfuric acid to form the ester. The process occurs at elevated temperatures to improve reaction speed.
- Water removal. The water generated during the reaction is removed through distillation to favor ester formation and prevent the reverse reaction.
- Cooling and neutralization. The reaction mixture is cooled, and excess acid is neutralized by adding a base, such as sodium hydroxide.
- Filtration. The mixture is filtered to remove the catalyst and other solid byproducts.
- Final distillation. The crude product is purified via vacuum distillation to isolate and collect diisopropyl sebacate.
- Quality control. The final product is tested to verify its purity, viscosity, and other physical properties to ensure it meets required standards.
What it is used for and where
Diisopropyl Sebacate is an ingredient found in cosmetic formulations due to its emollient and solvent properties. It provides a lightweight, non-greasy texture to skincare products, improving their application and feel. It is often used in creams, lotions, and sunscreens, where it helps evenly distribute active ingredients and leaves the skin soft and smooth.
Cosmetics - INCI Functions
- Skin conditioning agent - Emollient. Emollients have the characteristic of enhancing the skin barrier through a source of exogenous lipids that adhere to the skin, improving barrier properties by filling gaps in intercorneocyte clusters to improve hydration while protecting against inflammation. In practice, they have the ability to create a barrier that prevents transepidermal water loss. Emollients are described as degreasing or refreshing additives that improve the lipid content of the upper layers of the skin by preventing degreasing and drying of the skin. The problem with emollients is that many have a strong lipophilic character and are identified as occlusive ingredients; they are oily and fatty materials that remain on the skin surface and reduce transepidermal water loss. In cosmetics, emollients and moisturisers are often considered synonymous with humectants and occlusives.
- Plasticiser. Ingredient added to the formulation with the purpose of retaining fragrance and colour, increasing flexibility, flowability, deformability, durability of various ingredients allowing better processing. It softens and makes flexible synthetic polymers that otherwise could not be easily processed, stretched or deformed.
- Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
- Solvent. It is the substance for dissolving or dispersing surfactants, oils, dyes, flavourings, bactericidal preservatives in solution.In fact, it dissolves other components present in a cosmetic formulation. Solvents are generally liquid (aqueous and non-aqueous).
Cosmetic Applications
Emollient Effect. Helps make the skin soft and smooth to the touch, improving the product's feel on the skin.
Light and Non-Greasy. Provides hydration without leaving an oily residue, making it ideal for lotions, creams, and hair care products.
Facilitates Product Distribution. Enhances the glide and spreadability of cosmetic products on the skin, allowing for better application (1).
Texture Agent. Helps regulate the viscosity of cosmetic products, ensuring a homogeneous consistency.
Skin Compatibility. It is generally well tolerated by the skin and can be used even on sensitive skin.
Other Applications
Lubricants. Used as a lubricant in metalworking processes due to its low viscosity and stability.
Plastic Industry. Employed as a plasticizer to improve the flexibility of plastic materials.
Safety
Diisopropyl Sebacate, according to a study by the CIR Expert Panel, was found to be minimally irritating only to the eyes in laboratory animals and is therefore considered a safe ingredient (2).
Molecular Formula C16H30O4
Molecular Weight 286.41 g/mol
CAS 7491-02-3
UNII J8T3X564IH
EC Number 231-306-4
Synonyms:
diisopropylsebacate
di-isopropyl sebacate
IPSE
DERMOL DIPS
References_____________________________________________________________________
(1) Kurohane K, Kimura A, Terasawa R, Kobayashi K, Suzuki W, Matsuoka T, Imai Y. An Aliphatic Ester Diisopropyl Sebacate Exhibited an Adjuvant Effect on Fluorescein Isothiocyanate-Induced Contact Hypersensitivity Mouse Models. Biol Pharm Bull. 2018 Jan 1;41(1):147-150. doi: 10.1248/bpb.b17-00723.
Abstract. Alternative plasticizers have become more popular due to health concerns about phthalate esters. We demonstrated that phthalate esters enhanced skin sensitization to fluorescein isothiocyanate (FITC) in mouse contact hypersensitivity models. Alternative plasticizers have not been well studied as to their effect on the immune system. We previously found that diisopropyl adipate (DIPA), an aliphatic dicarboxylic acid ester, enhanced skin sensitization to FITC. Sebacate esters are also widely used as alternative plasticizers. Here we tested diisopropyl sebacate (DIPS), which has the same alcohol with an aliphatic dicarboxylic acid of longer chain, using BALB/c mice. The results showed that DIPS facilitated skin sensitization to FITC and increased FITC-presenting dendritic cell trafficking from the skin to draining lymph nodes. Furthermore, DIPS activated transient receptor potential ankyrin 1 (TRPA1). The latter feature has been commonly observed for phthalate esters and DIPA, which have adjuvant effects. In summary, the adjuvant effect of a sebacate ester was demonstrated in a mouse model.
(2) Fiume, M. M., Eldreth, H., Bergfeld, W. F., Belsito, D. V., Hill, R. A., Klaassen, C. D., ... & Andersen, F. A. (2012). Final report of the cosmetic ingredient review expert panel on the safety assessment of dicarboxylic acids, salts, and esters. International journal of toxicology, 31(4_suppl), 5S-76S.
Abstract. The CIR Expert Panel assessed the safety of dicarboxylic acids and their salts and esters as used in cosmetics. Most dicarboxylic acids function in cosmetics as pH adjusters or fragrance ingredients, but the functions of most of the salts in cosmetics are not reported. Some of the esters function as skin conditioning or fragrance ingredients, plasticizers, solvents, or emollients. The Expert Panel noted gaps in the available safety data for some of the dicarboxylic acid and their salts and esters in this safety assessment. The available data on many of the ingredients are sufficient, however, and similar structural activity relationships, biologic functions, and cosmetic product usage suggest that the available data may be extrapolated to support the safety of the entire group. The Panel concluded that the ingredients named in this report are safe in the present practices of use and concentration.
![]() Diisopropyl sebacate
Diisopropyl sebacate