Tioconazole is an antifungal chemical compound used to treat fungal infections of the skin and nails.
Chemical Industrial Synthesis Process
- Preparation of reagents. The main raw materials include 2,4-dichloroacetophenone, 1H-imidazole, and potassium thiocyanate.
- Synthesis of intermediate. The reaction begins with the condensation of 2,4-dichloroacetophenone with 1H-imidazole in the presence of a condensing agent such as aluminum chloride. This forms an important intermediate for subsequent synthesis steps.
- Formation of thiocyanate. The intermediate is then reacted with potassium thiocyanate in the presence of an appropriate solvent, such as dimethylformamide (DMF), to form the thiocyanate compound.
- Cyclization. The thiocyanate compound undergoes cyclization under controlled conditions of heat and pressure to form the basic structure of tioconazole.
- Purification. The crude tioconazole is purified to remove impurities and reaction byproducts using techniques such as crystallization, filtration, and chromatography.
- Stabilization. The purified tioconazole is stabilized to ensure its stability during transportation and storage, preventing degradation.
- Quality control. The tioconazole undergoes rigorous quality testing to ensure it meets standards for purity, efficacy, and safety. These tests include chemical analysis, spectroscopy, and microbiological testing.
A cosa serve e dove si usa
Tioconazole is an antifungal agent belonging to the azole derivatives class. It is effective against a wide range of fungal infections, including those causing ringworm, athlete's foot, and nail infections. This drug works by inhibiting the synthesis of ergosterol, a vital component of fungal cell membranes, thereby stopping the growth and reproduction of fungi. It is commonly used in creams, gels, and topical solutions. Tioconazole is valued for its effectiveness and its ability to quickly reduce the symptoms of fungal infections (1).
Cosmetica - Funzioni INCI
- Antimicrobial agent. This ingredient is able to suppress or inhibit the growth and replication of a broad spectrum of microorganisms such as bacteria, fungi and viruses by making the stratum corneum temporarily bactericidal and fungicidal (2).
Cosmetic Applications
Antifungal Treatment. Tioconazole is used in creams, gels, and lotions to treat fungal infections such as tinea pedis (athlete's foot), tinea cruris (jock itch), and tinea corporis (ringworm).
Prevention of Fungal Regrowth. It helps prevent the regrowth of fungi in treated areas, keeping the skin healthy.
Versatile Applications. It can be used in various skincare products designed to treat localized fungal infections (3).
Protective Effect. Provides long-lasting protection against the proliferation of fungi on the skin, reducing the risk of reinfections.
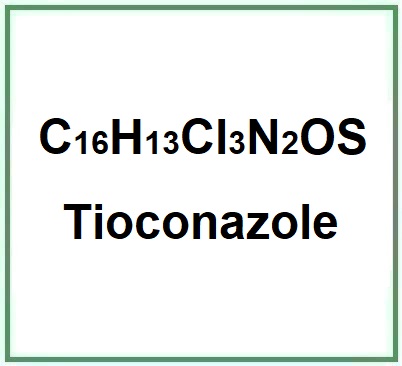
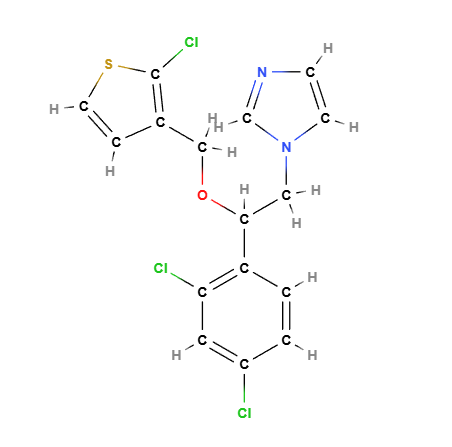
Molecular Formula C16H13Cl3N2OS
Molecular Weight 387.7 g/mol
CAS 65899-73-2
UNII S57Y5X1117
EC Number 265-973-8
Synonyms:
Vagistat
Mykontral
Monistat
Trosderm
Trosid
Trosyd
References_____________________________________________________________________
(1) Flores, F. C., Chiu, W. S., Beck, R. C., da Silva, C. B., & Delgado-Charro, M. B. (2018). Enhancement of tioconazole ungual delivery: Combining nanocapsule formulation and nail poration approaches. International Journal of Pharmaceutics, 535(1-2), 237-244.
(2) Clissold SP, Heel RC. Tioconazole. A review of its antimicrobial activity and therapeutic use in superficial mycoses. Drugs. 1986 Jan;31(1):29-51. doi: 10.2165/00003495-198631010-00003.
Abstract. Tioconazole is a substituted imidazole antimicrobial agent structurally related to other drugs in this group. It has been shown to have a broad spectrum of activity in vitro against dermatophytes and yeasts, as well as against some chlamydia, trichomonads and Gram-positive bacteria. Both open and controlled clinical trials have clearly demonstrated the efficacy and safety of topical preparations of tioconazole for treating superficial dermatophyte or yeast infections of the skin and vaginal candidiasis. In comparative studies it was at least as effective as alternative imidazole antifungal drugs, and in a few trials significantly greater efficacy has been reported for tioconazole, compared with clotrimazole, miconazole, econazole and systemic ketoconazole. Preliminary studies in other clinical areas suggest tioconazole may be useful for treating onychomycosis (in a special nail formulation), napkin-rash due to Candida albicans, impetigo, and vaginal trichomoniasis, although comparative studies are needed in each of these settings to clearly assess its relative place in therapy. Thus, tioconazole is an effective and well tolerated treatment for vaginal candidiasis and superficial fungal infections of the skin.
(3) Hay, R. J., Mackie, R. M., & Clayton, Y. M. (1985). Tioconazole nail solution—an open study of its efficacy in onychomycosis. Clinical and Experimental Dermatology, 10(2), 111-115.
Abstract. In view of the problems encountered in the treatment of onychomycosis with orally administered antifungal drugs, alternative forms of therapy arc needed. Tioconazole (28%) nail solution is a new topical preparation for use on infected nails. In this study 27 patients received treatment with tioconazole (28%) for up to 12 months. Six patients (22%) achieved complete clinical remission and were free of infection at follow-up, 3 months after therapy. They included infections caused by Trichophyton rubruni (4), Hendersonula toruloidea (1) and Acremonium (1). Apart from the latter, all infections responding to treatment were in the finger nails, even though three patients had active infection in the toe nails as well which did not respond to therapy. Significant improvements were recorded in a further 11 patients. They did not, however, achieve complete clinical and mycological recovery. The results indicate that cures of onychomycosis are possible after topical therapy, and further methods of using this form of treatment such as combined surgical and topical therapy are discussed.
![]() Tioconazole
Tioconazole