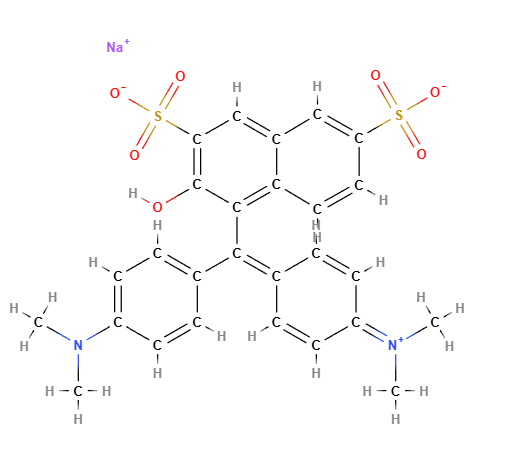
Acid green 50 is a chemical compound, synthetic green triphenylmethane dye (Triphenylmethane is a hydrocarbon), an ingredient listed in the Colour Index International. In the list of European food additives it is the colour E142 . It is also known as CI 44090, Green S.
Chemical name: sodium;4-[[4-(dimethylamino)phenyl]-(4-dimethylazaniumylidenecyclohexa-2,5-dien-1-ylidene)methyl]-3-hydroxynaphthalene-2,7-disulfonate
It appears in the form of a green powder

What it is used for and where
Cosmetics
Restricted cosmetic ingredient as IV/70 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: Hydrogen [4-[4-(dimethylamino)-.alpha.-(2-hydroxy-3,6-disulphonato-1-naphthyl)benzylidene]cyclohexa-2,5-dien-1-ylidene]dimethylammonium, monosodium salt
Cosmetics - INCI Functions
Colorant. This ingredient has the primary function of colouring the solution in which it is inserted in a temporary, semi-permanent or permanent manner, either alone or in the presence of the complementary components added for colouring.
Safety
E142 o Green S showed no adverse signs in studies at doses up to 500 mg per day in relation to body weight/day (1), but interesting activity against Plasmodium falciparum (Welch, 1897) the unicellular parasitic protozoan responsible for malaria (2).
Molecular Formula C27H25N2NaO7S2
CAS 3087-16-9
EC number 221-409-2
UNII 9B7E8Y9D0X
DTXSID4046577
Synonyms:
- CI 44090
- Green S
- Wool Green S
- Calocid Green S
- Lissamine Green B
- Acilan Green BS
- Food Green S
- Vondacid Green S
- Acid Leather Green S
- Erio Green S
- Acid Brilliant Green BS
References_____________________________________________________________________
(1) Moorhouse SR, Creasy DM, Gaunt IF. Three-generation toxicity study of rats ingesting Green S in the diet. Food Chem Toxicol. 1987 Dec;25(12):985-93. doi: 10.1016/0278-6915(87)90293-6.
Abstract. Green S was fed to rats of both sexes, over three generations, at dietary concentrations designed to provide daily intakes of 0, 50, 500 or 1000 mg Green S/kg body weight. At each generation, treated groups each consisted of 36 males and 36 females with 60 of each sex as controls. The F0 generation first received Green S as weanlings, but succeeding generations were exposed throughout life, including in utero, as treatment continued during gestation and lactation. There were no adverse effects of treatment on body-weight gain, food and water consumption or on the general condition of the animals. Green-coloured faeces were produced by all animals exposed to the colouring and pale green coloration of urine or the bladder was seen in a few animals at autopsy. The post-mortem examinations and organ weights of animals receiving up to 500 mg Green S/kg/day showed no adverse effects of treatment. At 1000 mg/kg/day findings related to treatment were increased spleen weight (both sexes) and increased kidney weight (male), but relevant histopathological changes were not seen in either of these organs. Caecal enlargement was the most consistent finding in animals receiving 500 or 1000 mg Green S/kg/day, but this was not considered to be a toxic effect. Reproductive performance and intra-uterine development were not affected by treatment despite green colouring being visible in the amniotic sacs of foetuses from dams given 500 or 1000 mg Green S/kg/day. Small differences in the degree of skeletal ossification of foetuses from F2 generation dams were not related to treatment. A slightly premature eruption of the incisors during postnatal development of treated animals was not considered to be an adverse effect. It is concluded that the no-untoward-effect level in this study is 500 mg Green S/kg body weight/day.
Brantom PG, Creasy DM, Gaunt IF. Long-term toxicity study of Green S in mice. Food Chem Toxicol. 1987 Dec;25(12):977-83. doi: 10.1016/0278-6915(87)90292-4.
Abstract. Groups of 105 (control) or 65 (treated) female CD-1 mice were mated one to one with equal numbers of males after both sexes had received diets containing 0 (control), 0.033, 0.33 or 0.66% Green S for 9 wk. The number of animals pregnant, the number of young born and the number surviving were similar in all groups. One male and one female from each litter were used to provide groups of 85 (control) or 50 (treated) mice of each sex for the long-term study. Treatment with Green S continued throughout pregnancy and rearing. Body weight and condition were regularly monitored for each animal throughout the study. Blood was examined from groups of 20 mice from the control and highest treatment groups at wk 14, 28 and 51 and from all survivors at the end of the study. A post-mortem examination was carried out on all animals in the long-term study and a full range of tissues was preserved and examined by light microscopy. Organ weights were recorded at the autopsy of all mice reaching the end of the study. No effects that could be attributed to treatment were seen in any of the observations. The no-untoward-effect level of Green S fed to mice for 2 yr is concluded to be 0.66% of the diet, equivalent to intakes of approximately 530 and 660 mg/kg body weight/day in males and females, respectively.
(2) Leba LJ, Popovici J, Estevez Y, Pelleau S, Legrand E, Musset L, Duplais C. Antiplasmodial activities of dyes against Plasmodium falciparum asexual and sexual stages: Contrasted uptakes of triarylmethanes Brilliant green, Green S (E142), and Patent Blue V (E131) by erythrocytes. Int J Parasitol Drugs Drug Resist. 2017 Dec;7(3):314-320. doi: 10.1016/j.ijpddr.2017.07.002.
![]() Acid green 50
Acid green 50