Aspartame (L-aspartyl-L-phenylalanine methyl ester) is a chemical compound formed from aspartic acid and phenylalanine.
Aspartame is an artificial sweetener used in low-calorie foods and beverages. It is known for its high sweetening power, approximately 200 times that of sugar, and its ability to provide sweetness without significant calories. Aspartame is widely used in the food and cosmetic industries to enhance the taste of low-calorie products.
Chemical Composition and Structure
Aspartame is a dipeptide composed of the amino acids phenylalanine and aspartic acid, with an added methyl group. Its unique chemical structure allows it to mimic the sweet taste of sugar while contributing negligible calories, as it is metabolized in minimal amounts.
Physical Properties
It appears as a white crystalline powder, odorless, water-soluble, and with limited thermal stability. For this reason, it is generally used in products at room temperature or refrigerated, rather than in baked or heated foods.
Production Process
Aspartame is produced through a chemical synthesis process that combines the amino acids phenylalanine and aspartic acid, followed by the addition of a methyl group. The compound is then purified and crystallized for food or industrial use.
Applications
Medical: Used in medications and dietary supplements to improve taste without increasing caloric intake, particularly useful for patients with diabetes or specific dietary regimens.
Cosmetics: Aspartame is used in oral care products, such as toothpaste and mouthwash, to enhance flavor without promoting tooth decay.
Food: It is widely used in diet beverages, chewing gum, low-calorie yogurts, and tabletop sweeteners.
Industry: Aspartame is used in various food and non-food applications to improve taste or reduce calorie content.
Environmental and Safety Considerations
Aspartame is generally recognized as safe (GRAS) when used within regulatory limits. However, individuals with phenylketonuria (PKU) must avoid aspartame due to its phenylalanine content. Its environmental impact is minimal, as it is produced in regulated facilities and is biodegradable.
Industrially it occurs as a fine white powder.

Studies
The history of aspartame dates back to 1965 when the American chemist and researcher James Schlatter, in an attempt to create a drug against gastric ulcer, chemically derived aspartame in an intermediate step of creating a tetrapeptide of the peptide hormones gastrin.
It is one of the most common sweeteners and on which have been focused countless scientific researches to establish the risks connected to the consumption of this product which is included in many food products: diet drinks, chewing gums, yogurt, desserts, vitamins, medicines. It is recommended to people with diabetes as a substitute of sugar. It has about 200 times the sweetening power of sugar.
In the European Union it is labeled as E951 in the list of european food additives as a sweetener and it is considered by Efsa (European Food Safety Authority), safe for human health.
The acceptable daily intake of aspartame recommended by the FDA is 50 mg/kg per day in the United States and 40 mg/kg per day in the European Union (EFSA).
Researchers from the Schiller Institute for Integrated Science and Society, Boston, based on the 1997 toxicological studies of the Ramazzini Institute (an independent non-profit research laboratory based in Bologna) carried out on laboratory animals, concluded that aspartame is a chemical carcinogen in rodents even at the dose of 100 mg/kg body weight, a relatively low dose exposure dangerously close to the current permissible daily intake (ADI) levels in the USA and the European Union (1).
High-intensity sweeteners are considered potential organic contaminants due to their widespread use in food, drugs and healthcare products. They are introduced into the environment by different pathways and find their way into water (2).
Aspartame produces phenylalanine which can be dangerous for patients with phenylketonuria.
For more information:
Aspartame studies
Typical optimal characteristics of the commercial product Aspartame
| Appearance | Powder fine white |
| Density | 1.3±0.1 g/cm3 |
| Alpha | 15.5 º (c=4, 15N formic acid) |
| Melting Point | 242-248 °C |
| Boiling Point | 535.8±50.0 °C at 760 mmHg |
| Flash Point | 277.8±30.1 °C |
| 14.5 ° (C=4, 15mol/L Formic Acid) |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Storage | 2-8°C |
| Solubility | Sparingly soluble or slightly soluble in water and in ethanol (96 per cent), practically insoluble in hexane and in methylene chloride. |
| Pka | pKa 3.19±0.01 (H2O t=25.0 I=0.100(NaCl))(Approximate);7.87±0.02(H2O t=25.0 I=0.100(NaCl))(Approximate) |
 |  |
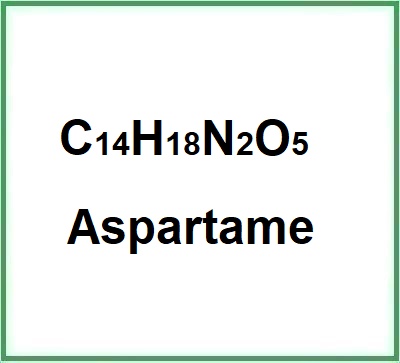
- Molecular Formula: C14H18N2O5
- Linear Formula:HOOCCH2CH(NH2)CONHCH(CH2C6H5)COOCH3
- Molecular Weight: 294.307 g/mol
- CAS: 22839-47-0 7421-84-3
- EC Number: 245-261-3 616-062-2
- UNIII: Z0H242BBR1
- Beilstein Registry Number:2223850
- MDL number: MFCD00002724
- PubChem Substance ID 24870725
- InChI=1S/C14H18N2O5/c1-21-14(20)11(7-9-5-3-2-4-6-9)16-13(19)10(15)8-12(17)18/h2-6,10-11H,7-8,15H2,1H3,(H,16,19)(H,17,18)/t10-,11-/m0/s1
- InChl Key IAOZJIPTCAWIRG-QWRGUYRKSA-N
- SMILES COC(=O)C(CC1=CC=CC=C1)NC(=O)C(CC(=O)O)N
- IUPAC (3S)-3-amino-4-[[(2S)-1-methoxy-1-oxo-3-phenylpropan-2-yl]amino]-4-oxobutanoic acid
- ChEBI 2877
Synonyms:
- Nutrasweet
- L-Aspartyl-L-phenylalanine methyl ester
- Asp-phe-ome
- Aspartylphenylalanine methyl ester
- Asp-Phe methyl ester
- Methyl aspartylphenylalanate
- Aspartam
- Dipeptide sweetener
- Aspartamum
- 1-Methyl N-L-alpha-aspartyl-L-phenylalanate
- Aspartamo
- 3-amino-3-[(1-methoxycarbonyl-2-phenyl-ethyl)carbamoyl]propanoic acid
References_____________________________________________________________________
(1) Landrigan PJ, Straif K. Aspartame and cancer - new evidence for causation. Environ Health. 2021 Apr 12;20(1):42. doi: 10.1186/s12940-021-00725-y.
Abstract. Background: Aspartame is one of the world's most widely used artificial sweeteners and is an ingredient in more than 5000 food products globally. A particularly important use is in low-calorie beverages consumed by children and pregnant women. The Ramazzini Institute (RI) reported in 2006 and 2007 that aspartame causes dose-related increases in malignant tumors in multiple organs in rats and mice. Increased cancer risk was seen even at low exposure levels approaching the Acceptable Daily Intake (ADI). Prenatal exposures caused increased malignancies in rodent offspring at lower doses than in adults. These findings generated intense controversy focused on the accuracy of RI's diagnoses of hematopoietic and lymphoid tissue tumors (HLTs). Critics made the claim that pulmonary lesions observed in aspartame-exposed animals were inflammatory lesions caused by Mycoplasma infection rather than malignant neoplasms. Interpretation: These new findings confirm that aspartame is a chemical carcinogen in rodents. They confirm the very worrisome finding that prenatal exposure to aspartame increases cancer risk in rodent offspring. They validate the conclusions of the original RI studies. These findings are of great importance for public health. In light of them, we encourage all national and international public health agencies to urgently reexamine their assessments of aspartame's health risks - especially the risks of prenatal and early postnatal exposures. We call upon food agencies to reassess Acceptable Daily Intake (ADI) levels for aspartame. We note that an Advisory Group to the International Agency for Research on Cancer has recommended high-priority reevaluation of aspartame's carcinogenicity to humans.
(2) Arbeláez P, Borrull F, Pocurull E, Marcé RM. Determination of high-intensity sweeteners in river water and wastewater by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2015 May 8;1393:106-14. doi: 10.1016/j.chroma.2015.03.035.
![]() Aspartame
Aspartame