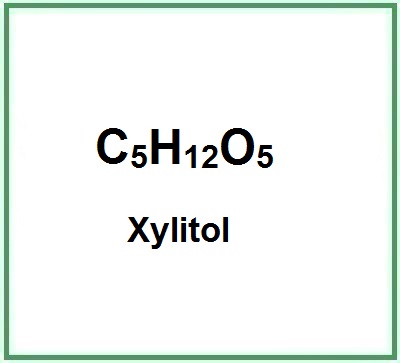
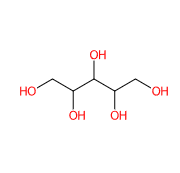
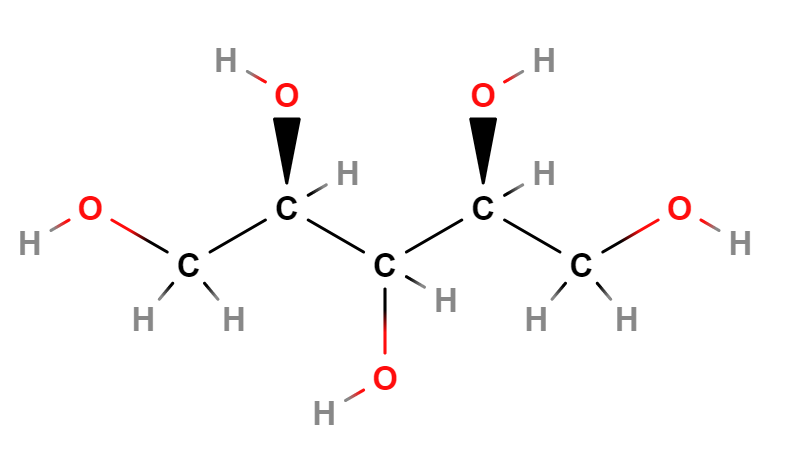
Xylitol is a sugar alcohol with five carbon atoms and ethanol and water are used for its industrial extraction. It is produced naturally by mammalian metabolism.
Description of raw materials used in production:
Xylan extracted from lignocellulosic biomass such as wheat straw, wood waste, and corn cobs. Xylan is a polysaccharide that serves as a precursor for xylitol.
Hydrogen used in the hydrogenation process to reduce the functional groups of xylan into xylitol.
Industrial Chemical Synthesis
- Preparation: The mixture is prepared by hydrolyzing xylan to obtain a sugar solution, primarily xylose.
- Hydrogenation: The xylose solution is exposed to hydrogen in the presence of a catalyst, such as ruthenium or nickel. This process occurs under high pressure and temperature conditions.
- Cooling and Separation: After hydrogenation, the mixture is cooled. Xylitol separates from the solution as a viscous liquid.
- Purification: The xylitol solution is purified to remove the catalyst and other impurities. This can include filtration and crystallization.
- Concentration: The purified xylitol solution is concentrated to remove excess water, achieving a desired concentration.
- Crystallization and Drying: Xylitol can be further crystallized and dried to obtain a solid form.
It appears as a white crystalline powder.

What it is used for and where
Food
It is an artificial sweetener of vegetable origin that replaces sugar, labeled with the number E967 in the list of food additives, it is called "the sugar of wood" as it is made from some types of trees and corn. It is produced by the hydrogenation of Xylose in conditions of high temperature (80-140 ° C) and high pressure (up to 50 atm) (1).
It belongs to polyalcohols that have the characteristic of contrasting with acids that affect tooth enamel. This is why it is used in chewing gum and in the food and pharmaceutical fields.
After 1960, Xylitol has become a common ingredient in sugar-free baked goods, anticorrosive toothpaste and mouthwashes, oral care products and diabetic food.
Excessive intake may cause laxative effects.
Cosmetics
Anti-sebum. Controls and reduces emissions from the sebaceous glands, which are responsible for greasy, enlarged pores in the skin, where it occurs, particularly on the forehead, cheeks, nose and hair. Adjuvant in the treatment of acne.
Deodorant agent. When substances that give off an unpleasant odour are included in cosmetic formulations (typical examples are methyl mercaptan and hydrogen sulphide derived from garlic), deodorants attenuate or eliminate the unpleasant exhalation.
Flavoring agent. The purpose of this ingredient is to modify the solution to impart a certain flavour. Natural flavouring extracts are rather expensive, so the cosmetic and pharmaceutical industries resort to synthesised substances that have sensory characteristics mostly similar to natural flavourings or are naturally equivalent. This ingredient is isolated through chemical processes or is synthesised from chemicals. It is also referred to as Aroma.
Humectant. Hygroscopic compound used to minimise water loss in the skin and to prevent it from drying out by facilitating faster and greater absorption of water into the stratum corneum of the epidermis. The epidermis is the most superficial of the three layers that make up human skin (epidermis, dermis and hypodermis) and is the layer that maintains hydration in all three layers. In turn, the epidermis is composed of five layers: horny, the most superficial, granular, spinous, shiny, and basal. Humectants have the ability to retain the water they attract from the air in the stratum corneum and have the function of moisturising the skin. They are best used before emollients, which are oil-based.
Skin conditioning agent - Humectant. Humectants are hygroscopic substances used to minimise water loss in the skin and to prevent it from drying out by facilitating faster and greater absorption of water into the stratum corneum of the epidermis. The epidermis is the most superficial of the three layers that make up the human skin (epidermis, dermis and hypodermis) and is the layer that maintains hydration in all three layers. In turn, the epidermis is composed of five layers: corneum, the most superficial, lucidum, granulosum, spinosum and basale. Humectants have the ability to retain in the stratum corneum the water they attract from the air and have the function of moisturising the skin. It is better to use them before emollients that are oil-based.
Medical
It is used in the prevention of acute otitis media, respiratory diseases, parenteral nutrition, atopic dermatitis, wound repair, gastrointestinal infections, osteoporosis, anti-aging and inflammatory processes (2) and in food for diabetics.
The most relevant studies on this ingredient have been selected with a summary of their contents:
Xylitol studies
Typical commercial product characteristics Xylitol
| Appearance | White powder |
Boiling Point
| 494.5±40.0°C at 760 mmHg |
Melting Point
| 94-97°C(lit.) |
Flash Point
| 261.9±21.9°C |
Loss on Drying
| 5% |
Sulphated Ash
| 5% |
Conductivity ash
| 0.007 |
Particle size > 2.4mm
| 0 |
Heavy Metal
| 5ppm |
| As | 2ppm |
| Lead | <0.3 |
| Nickel | <1 |
Total Plate
| 1000/g Max |
Yeast & Mold
| 100/g Max |
pH 10% w/v
| 5.5 |
Other polyols HPLC
| <0.4 |
Reducing sugars
| 0.03 |
- Molecular Formula C5H12O5
- Condensed Formula HOCH2[CH(OH)]3CH2OH
- Molecular Weight 152.15
- MExact Mass 152.068466
- CAS 87-99-0
- UNII VCQ006KQ1E
- IUPAC (2S,4R)-pentane-1,2,3,4,5-pentol
- InChI=1S/C5H12O5/c6-1-3(8)5(10)4(9)2-7/h3-10H,1-2H2/t3-,4+,5?
- InChl Key HEBKCHPVOIAQTA-NGQZWQHPSA-N
- SMILES C(C(C(C(CO)O)O)O)O
- EC Number: 207-685-7 201-788-0
- DSSTox Substance ID: DTXSID7042514
- MDL number MFCD00064292
- PubChem Substance ID 24902140
- Beilstein 1720523
- NACRES: NA.25
- RTECS ZF0800000
Synonyms:
- Xylite
- ribitol
- D.ribitol
- D-Xylitol
- (2S,4R)-pentane-1,2,3,4,5-pentol
- 1,2,3,4,5-Pentahydroxypentane
References________________________________
(1) Granström TB, Izumori K, Leisola M. A rare sugar xylitol. Part II: biotechnological production and future applications of xylitol. Appl Microbiol Biotechnol. 2007
Wisniak J, Hershkowitz M, Leibowitz R, Stein S. Hydrogenation of xylose to xylitol. Ind Eng Chem Res. 1974
(2) Da Silva, S.S. and Chandel, A.K. eds., 2012. D-xylitol: fermentative production, application and commercialization. Springer Science & Business Media.
![]() Xylitol
Xylitol