![]() Brucine
Brucine
Rating : 5
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
0 pts from admin
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Brucine by admin (19549 pt) | 2024-Sep-21 11:22 |
| Read the full Tiiip | (Send your comment) |
Brucine is a bitter alkaloid derived from the seeds of the Strychnos nux-vomica tree, which is native to tropical Asia. Known for its potent bitterness, brucine has been historically utilized in traditional medicine for its stimulant and digestive properties. In cosmetic formulations, it is valued for its astringent effects and potential to stimulate microcirculation, making it beneficial for skin toning and tightening.
Chemical Composition and Structure
The chemical composition of Brucine includes:
- Brucine: An alkaloid with the molecular formula C23H26N2O4, featuring a complex bicyclic structure that is closely related to strychnine, another alkaloid.
Structurally, brucine consists of a tetracyclic framework with a methoxy group and an amine, which contribute to its unique biological activity and bitterness. Its specific stereochemistry plays a crucial role in its pharmacological effects.
Physical Properties
Appearance: Typically a white to pale yellow crystalline powder.
Solubility: Soluble in organic solvents like alcohol; slightly soluble in water, which may limit its use in certain formulations.
pH: Neutral to slightly acidic, depending on the formulation.
Odor: Odorless, making it suitable for a variety of cosmetic applications.
Stability: Stable under normal storage conditions; however, it should be protected from light and moisture to maintain potency.
Production Process
Extraction: Brucine is extracted from the seeds of Strychnos nux-vomica using methods such as cold maceration or solvent extraction. The extraction process typically involves soaking the seeds in a solvent to dissolve the alkaloids.
Purification: The extract is then subjected to purification techniques such as filtration, crystallization, or chromatography to isolate brucine from other components and impurities, ensuring a high-quality product.
Formulation: Purified brucine is incorporated into various herbal and cosmetic products, often combined with other ingredients to enhance efficacy and stability.
Applications
Medical: Brucine has been used in traditional medicine as a tonic and stimulant, particularly for digestive health. It is known to have potential effects on the central nervous system and may be used in very small doses for therapeutic purposes.
Cosmetics: In skincare, brucine is included for its astringent properties, helping to tighten and tone the skin. It may also enhance the absorption of other active ingredients, improving overall product efficacy.
INCI Functions:
Brucin is a substance. In cosmetics it is not used directly but in the form of Brucin sulfate as denaturant.
Denaturant. It makes cosmetics unpalatable. It is sometimes added to cosmetics containing ethyl alcohol to make it unsuitable for ingestion. The ionic or polar molecules of this ingredient included in formulations that interact with protein groups, modulate the properties of the solution to suit specific needs.
Food: Due to its toxicity at high doses, brucine is not typically used in food products. It is primarily recognized for its medicinal applications rather than culinary uses.
Industrial Uses: Occasionally, brucine is employed in pest control formulations as a natural insecticide due to its bitter taste, which can deter pests.
Environmental and Safety Considerations
The Panel acknowledged that the available data were insufficient to support the safety of Quassin, Brucin and Brucin sulfate, Alcohol denatured with these denaturants, or SD Alcohol 39 and SD Alcohol 40, and for the Panel to reach a conclusion for these denaturants, more data are needed. (2).
Brucin and can be toxic in certain doses, leading to potential side effects such as nausea or neurological symptoms. It may also cause skin irritation in sensitive individuals. Responsible sourcing and formulation practices are crucial to ensure that the ingredient is sustainably produced and free from harmful contaminants. Users should be informed of its potency and potential side effects, also in cosmetic applications.
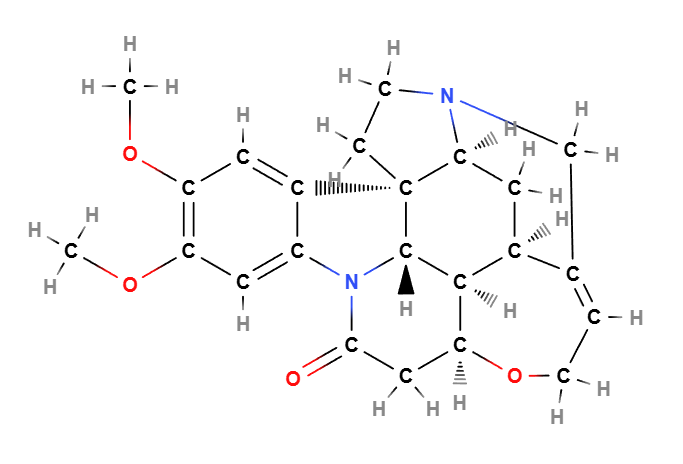 |  |
Molecular Formula C23H26N2O4
Molecular Weight 394.5 g/mol
CAS 357-57-3
UNII 6NG17YCK6H
EC Number 206-614-7
DTXSID2024662
Synonyms:
Brucinum
2,3-Dimethoxystrychnine
References__________________________________________________________________________
(1) Noman M, Qazi NG, Rehman NU, Khan AU. Pharmacological investigation of brucine anti-ulcer potential. Front Pharmacol. 2022 Aug 17;13:886433. doi: 10.3389/fphar.2022.886433. PMID: 36059979; PMCID: PMC9429807.
Yu G, Qian L, Yu J, Tang M, Wang C, Zhou Y, Geng X, Zhu C, Yang Y, Pan Y, Shen X, Tang Z. Brucine alleviates neuropathic pain in mice via reducing the current of the sodium channel. J Ethnopharmacol. 2019 Apr 6;233:56-63. doi: 10.1016/j.jep.2018.12.045.
Xu MR, Wei PF, Suo MZ, Hu Y, Ding W, Su L, Zhu YD, Song WJ, Tang GH, Zhang M, Li P. Brucine Suppresses Vasculogenic Mimicry in Human Triple-Negative Breast Cancer Cell Line MDA-MB-231. Biomed Res Int. 2019 Jan 6;2019:6543230. doi: 10.1155/2019/6543230.
Abstract. Vasculogenic mimicry (VM) with the pattern of endothelial independent tubular structure formation lined by aggressive tumor cells mimics regular tumor blood vessels to ensure robust blood supply and correlates with the proliferation, invasion, metastasis, and poor prognosis of malignant tumors, which was demonstrated to be a major obstacle for resistance to antiangiogenesis therapy. Therefore, it is urgent to discover methods to abrogate the VM formation of tumors, which possesses important practical significance for improving tumor therapy. Brucine is a traditional medicinal herb extracted from seeds of Strychnos nux-vomica L. (Loganiaceae) exhibiting antitumor activity in a variety of cancer models. In the present study, the effect of brucine on vasculogenic mimicry and the related mechanism are to be investigated. We demonstrated that, in a triple-negative breast cancer cell line MDA-MB-231, brucine induced a dose-dependent inhibitory effect on cell proliferation along with apoptosis induction at higher concentrations. The further study showed that brucine inhibited cell migration and invasion with a dose-dependent manner. Our results for the first time indicated that brucine could disrupt F-actin cytoskeleton and microtubule structure, thereby impairing hallmarks of aggressive tumors, like migration, invasion, and holding a possibility of suppressing vasculogenic mimicry. Hence, the inhibitory effect of brucine on vasculogenic mimicry was further verified. The results illustrated that brucine significantly suppressed vasculogenic mimicry tube formation with a dose-dependent effect indicated by the change of the number of tubules, intersections, and mean length of tubules. The in-depth molecular mechanism of vasculogenic mimicry suppression induced by brucine was finally suggested. It was demonstrated that brucine inhibited vasculogenic mimicry which might be through the downregulation of erythropoietin-producing hepatocellular carcinoma-A2 and matrix metalloproteinase-2 and metalloproteinase-9.
(2) Cosmetic Ingredient Review Expert Panel. Final report of the safety assessment of Alcohol Denat., including SD Alcohol 3-A, SD Alcohol 30, SD Alcohol 39, SD Alcohol 39-B, SD Alcohol 39-C, SD Alcohol 40, SD Alcohol 40-B, and SD Alcohol 40-C, and the denaturants, Quassin, Brucine Sulfate/Brucine, and Denatonium Benzoate. Int J Toxicol. 2008;27 Suppl 1:1-43. doi: 10.1080/10915810802032388.
Abstract. Alcohol Denat. is the generic term used by the cosmetics industry to describe denatured alcohol. Alcohol Denat. and various specially denatured (SD) alcohols are used as cosmetic ingredients in a wide variety of products. Many denaturants have been previously considered, on an individual basis, as cosmetic ingredients by the Cosmetic Ingredient Review (CIR) Expert Panel, whereas others, including Brucine and Brucine Sulfate, Denatonium Benzoate, and Quassin, have not previously been evaluated. Quassin is a bitter alkaloid obtained from the wood of Quassia amara. Quassin has been used as an insect antifeedant and insecticide and several studies demonstrate its effectiveness. At oral doses up to 1000 mg/kg using rats, Quassin was not toxic in acute and short-term tests, but some reversible piloerection, decrease in motor activity, and a partial loss of righting reflex were found in mice at 500 mg/kg. At 1000 mg/kg given intraperitoneally (i.p.), all mice died within 24 h of receiving treatment. In a cytotoxicity test with brine shrimp, 1 mg/ml of Quassin did not possess any cytotoxic or antiplasmodial activity. Quassin administered to rat Leydig cells in vitro at concentrations of 5-25 ng/ml inhibited both the basal and luteinizing hormone (LH)-stimulated testosterone secretion in a dose-related fashion. Quassin at doses up to 2.0 g/kg in drinking water using rats produced no significant effect on the body weights, but the mean weights of the testes, seminal vesicles, and epididymides were significantly reduced, and the weights of the anterior pituitary glands were significantly increased. The sperm counts and levels of LH, follicle-stimulating hormone (FSH), and testosterone were significantly lower in groups treated with Quassin. Brucine is a derivative of 2-hydroxystrychnine. Swiss-Webster mice given Brucine base, 30 ml/kg, had an acute oral LD(50) of 150 mg/kg, with central nervous system depression followed by convulsions and seizures in some cases. In those animals that died, respiratory arrest was the cause. The acute i.p. LD(50) for 15 ml/kg of Brucine base was 62.0 mg/kg, with central nervous system depression prior to the onset of convulsions, just as with oral Brucine. The acute intravenous (i.v.) LD(50) was 12.0 mg/kg. Brucine was nonmutagenic in an Ames assay at levels up to 6666 mu g/plate, with and without metabolic activation. In a repeat-insult patch test, for a hair care product containing 47% SD Alcohol 40 (95%), it was reported that Brucine Sulfate may be considered a nonprimary irritant and a nonprimary sensitizer. Three different sunscreen products (35% SD Alcohol 40-B, 72.4% SD Alcohol 40, and 74.5% SD Alcohol 40) did not show any signs of photoallergy in human subjects. Also, these three formulas did not exhibit any evidence of phototoxicity in humans. Denatonium Benzoate is a bitter substance detectable at a concentration of 10 ppb, discernibly bitter at 50 ppb, and unpleasantly bitter at 10 ppm. The distribution of topically applied lidocaine, a topical anesthetic chemically related to Denatonium Benzoate demonstrated that virtually no lidocaine appears in the plasma, suggesting that the larger Denatonium Benzoate molecule also would have little or no systemic exposure. Denatonium Benzoate (0.1%) did not show adverse effects in 10 rats in an acute inhalation toxicity test and 0.005% to 0.05% was nonirritating to ocular mucosa in 6 albino rabbits. The acute oral LD(50) for the male rats was 640 mg/kg and for females, 584 mg/kg. The LD(50) for the male rabbits was 508 mg/kg and for the female rabbits, 640 mg/kg. In two chronic toxicity studies, Denatonium Benzoate was administered (by gavage) at 1.6, 8, and 16 mg/kg/day, one using cynomologus monkeys and the other rats, resulted in no compound-related toxicity. The toxicity of SD Alcohols has also been tested, with implications for the particular denaturant used. An irritation test of 55.65% SD Alcohol 40-B denatured with Denatonium Benzoate using rabbits produced minimal effects. A spray formula containing 12% SD Alcohol 40-B was found to be nonirritating when evaluated for vaginal mucosal irritation in New Zealand white rabbits. Cosmetic formulations containing SD Alcohol 40-B (denatured with Denatonium Benzoate) were not sensitizers in repeated insult patch tests. A gel formula containing 29% SD Alcohol 40-B and a spray liquid containing 12% SD Alcohol 40-B did not induce photoallergy, dermal sensitization, or phototoxic response in human subjects. Although the absorption of ethanol (aka Alcohol for purposes of cosmetic ingredient labeling) occurs through skin, ethanol does not appear to affect the integrity of the skin barrier nor reach a very high systemic concentration following dermal exposure. Ethanol may be found in the bloodstream as a result of inhalation exposure and ingestion. Topically applied, ethanol can act as a penetration enhancer. Most of the systemic toxicity of ethanol appears to be associated with chronic abuse of alcohol. Although ethanol is denatured to make it unfit for consumption, there have been reports of intentional and unintentional consumption of products containing denatured alcohol. Ethanol is a reproductive and developmental toxicant. Ethanol is genotoxic in some test systems and it has been proposed that the genotoxic effects of ethanol are mediated via its metabolite, acetaldehyde. A brief summary is provided of the effects of chronic ingestion of alcohol including intoxication, liver damage, brain damage, and possible carcinogenicity. The CIR Expert Panel recognizes that certain ingredients in this group are reportedly used in a given product category, but the concentration of use is not available. Because dermal application or inhalation of cosmetic products containing these ingredients will not produce significant systemic exposure to ethanol, the CIR Expert Panel concluded that safety of the ingredients should be predicated on the safety of the denaturants used. The Panel considered that the adverse effects known to be associated with Alcohol ingestion included in this safety assessment do not suggest a concern for Alcohol Denat. or SD Alcohols because of the presence of the denaturants, which are added for the express purpose of making the Alcohol unpotable. The CIR Expert Panel has previously conducted safety assessments of t-Butyl Alcohol, Diethyl Phthalate, Methyl Alcohol, Salicylic Acid, Sodium Salicylate, and Methyl Salicylate, in which each was affirmed safe or safe with qualifications. Given their use as denaturants are at low concentrations of use in Alcohol, the CIR Expert Panel determined that Alcohol Denat. denatured with t-Butyl Alcohol, Diethyl Phthalate, Methyl Alcohol, Salicylic Acid, Sodium Salicylate, and Methyl Salicylate is safe as used in cosmetic formulations with no qualifications. Likewise, because they are denatured with either t-Butyl Alcohol, Diethyl Phthalate, or Methyl Alcohol, SD Alcohols 3-A, 30, 39-B, 39-C, and 40-C all are considered safe as used. The Panel considered the available data for Denatonium Benzoate and SD Alcohol 40-B to be sufficient to support the safety of these ingredients in cosmetics. Denatonium Benzoate is sufficiently bitter that it is an effective denaturant at only 0.0006%. The Panel recognized that data on dermal penetration of Denatonium Benzoate were not available, but considered that the available data on lidocaine, a smaller structurally related chemical, indicates that dermal exposure does not result in measurable systemic exposure. The available data, however, were not sufficient to support the safety of Quassin, Brucine, and Brucine Sulfate, Alcohol Denat. denatured with those denaturants, or SD Alcohol 39 and SD Alcohol 40 (SD Alcohols denatured with Quassin, Brucine, and/or Brucine Sulfate), and in order for the Expert Panel to reach a conclusion for these denaturants, additional data are needed
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2024-09-21 10:49:38 | Chemical Risk: |

