![]() Levocarnitine
Levocarnitine
Rating : 8
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
0 pts from admin
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Levocarnitine by admin (19557 pt) | 2025-Jan-04 16:51 |
| Read the full Tiiip | (Send your comment) |
Levocarnitine, also known as L-carnitine, is a naturally occurring amino acid derivative crucial for cellular energy production. Widely used in cosmetics, pharmaceuticals, and dietary supplements, it is valued for its antioxidant, moisturizing, and skin-conditioning properties. Its ability to support cellular metabolism and enhance skin elasticity makes it a key ingredient in anti-aging and revitalizing skincare formulations.
Chemical Composition and Structure
Levocarnitine is a quaternary ammonium compound with the chemical formula C7H15NO3.
Core Structure:
- Derived from lysine and methionine, it contains a hydroxyl group, a carboxyl group, and a quaternary ammonium group.
- Its zwitterionic nature contributes to excellent solubility in water and high biocompatibility.
Biological Role:
- Acts as a carrier molecule in mitochondrial energy production, transporting long-chain fatty acids for beta-oxidation.
Physicochemical Properties
- Appearance: White crystalline powder.
- Odor: Odorless.
- Solubility: Highly soluble in water; slightly soluble in alcohol.
- Stability: Stable under normal conditions; sensitive to strong acids, bases, and high temperatures.
- pH: Neutral to slightly acidic in aqueous solutions.
Production Process
Biosynthesis (Natural Source):
- Naturally synthesized in the liver and kidneys from lysine and methionine in the presence of vitamin C, iron, and B-complex vitamins.
Synthetic Production:
- Commercially manufactured through a chemical or biotechnological process involving the reaction of lysine derivatives with other organic compounds.
Purification:
- The final product undergoes rigorous purification to achieve pharmaceutical or cosmetic-grade quality.
Applications
Medical Applications
- Energy Metabolism: Used as a dietary supplement to support cellular energy production and treat carnitine deficiency.
- Therapeutic Use: Found in formulations to address conditions like chronic fatigue and muscle weakness.
- Skin Repair: Topical formulations enhance skin repair and recovery after stress or damage.
Carnitine is synthesised in many eukaryotic organisms and its biosynthesis is initiated by the methylation of lysine. It plays a key role in the metabolism of fatty acids in the mitochondria.
Carnitine, however, is not considered an essential nutrient and is present in many foods, mainly those from animal sources. Lysine and methionine are necessary ingredients for carnitine biosynthesis.
All body tissues can produce deoxy-carnitine but, in humans, the enzyme that enables the hydroxylation of deoxy-carnitine to carnitine is only found in the liver, brain and kidneys.
Cosmetics
Anti-Aging:
- Boosts cellular energy production, supporting collagen synthesis and reducing signs of aging like wrinkles and fine lines.
- Improves skin elasticity and firmness.
Moisturization:
- Enhances water retention in the skin, providing long-lasting hydration.
Skin Revitalization:
- Promotes cell turnover and reduces dullness, leaving the skin refreshed and radiant.
Haircare:
- Used in shampoos and conditioners to improve scalp health and strengthen hair follicles.
INCI Functions
Anti-static agent. Static electricity build-up has a direct influence on products and causes electrostatic adsorption. The antistatic ingredient reduces static build-up and surface resistivity on the surface of the skin and hair.
Cleansing agent. Ingredient that cleanses skin without exploiting the surface-active properties that produce a lowering of the surface tension of the stratum corneum.
Skin conditioning agent. It is the mainstay of topical skin treatment as it has the function of restoring, increasing or improving skin tolerance to external factors, including melanocyte tolerance. The most important function of the conditioning agent is to prevent skin dehydration, but the subject is rather complex and involves emollients and humectants that can be added in the formulation.
Hair conditioning agent. A significant number of ingredients with specific and targeted purposes can co-exist in a hair shampoo: cleansers, conditioners, thickeners, mattifying agents, sequestering agents, fragrances, preservatives, special additives. However, the indispensable ingredients are the cleansers and conditioners as they are necessary and sufficient for hair cleansing and manageability. The others have commercial and non-essential accessory acts such as: appearance, perfume, colouring, etc. Hair conditioning agents have the task of increasing shine, manageability and volume, and reducing static electricity, especially after treatments such as colouring, ironing, waving, drying and brushing. They are, in practice, dispersants that may contain cationic surfactants, thickeners, emollients, polymers. The typology of hair conditioning agents includes: intensive conditioners, instant conditioners, thickening conditioners, drying conditioners.
Surfactant - Cleansing agent. Cosmetic products used to cleanse the skin utilise the surfactant action that produces a lowering of the surface tension of the stratum corneum, facilitating the removal of dirt and impurities.
Surfactant - Foam booster. This has the function of introducing gas bubbles into the water for a purely aesthetic factor, which does not affect the cleansing process, but only satisfies the commercial aspect of the cleanser by helping to spread the cleanser on the hair. This helps in the commercial success of a shampoo formulation. Since sebum has an inhibiting action on the bubble, more foam is produced in the event of a second shampoo.
Viscosity-increasing agent, aqueous. Since viscosity is important to increase the chemical and physical stability of the product, Viscosity Enhancing Agent, aqueous is an important dosage factor in gels, suspensions, emulsions, solutions. Increasing viscosity makes formulations less sedimentary and more homogeneously thickened.
Industrial Applications
- Used in formulations targeting high-performance skincare and haircare.
- Found in clean and natural beauty products for its biocompatibility and multifunctional benefits.
Environmental and Safety Considerations
Biodegradability:
- As a naturally occurring compound, Levocarnitine is biodegradable and poses minimal environmental risk.
Safety Profile:
- Non-toxic and well-tolerated at typical cosmetic and therapeutic concentrations.
- Rare cases of mild irritation may occur with very high concentrations.
Sustainability:
- Production from natural sources or biotechnology ensures an eco-friendly approach.
Conclusion
Levocarnitine is a versatile and effective ingredient in both cosmetic and medical formulations. Its ability to enhance energy metabolism, improve skin hydration, and support anti-aging treatments makes it a valuable addition to skincare and haircare products. Its excellent biocompatibility and minimal environmental impact further enhance its appeal in modern formulations.
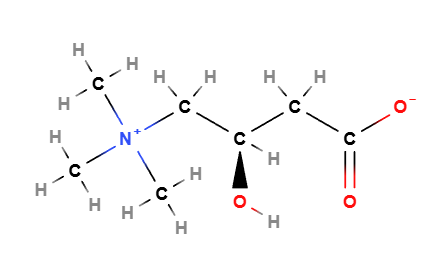 |  |
Molecular Formula C7H15NO3
Molecular Weight 161.20 g/mol
CAS 541-15-1
UNII 0G389FZZ9M
EC Number 208-768-0
CHEMBL1149
DTXSID4023208
Synonyms:
L Carnitine
Levocarnitine
References__________________________________________________________________________
Pistone G, Marino A, Leotta C, Dell'Arte S, Finocchiaro G, Malaguarnera M. Levocarnitine administration in elderly subjects with rapid muscle fatigue: effect on body composition, lipid profile and fatigue. Drugs Aging. 2003;20(10):761-7. doi: 10.2165/00002512-200320100-00004.
Abstract. Aim: Levocarnitine is an important contributor to cellular energy metabolism. This study aims to evaluate the effects of levocarnitine supplementation on body composition, lipid profile and fatigue in elderly subjects with rapid muscle fatigue. Method: This was a placebo-controlled, randomised, double-blind, two-phase study. Eighty-four elderly subjects with onset of fatigue following slight physical activity were recruited to the study. Prior to randomisation all patients entered a 2-week normalisation phase where they were given an 'ad libitum'diet, according to the National Cholesterol Education Program (Step 2). Subjects were asked to record their daily food intake every 2 days. Before the 30-day treatment phase, subjects were randomly assigned to two groups (matched for male/female ratio, age and body mass index). One group received levocarnitine 2g twice daily (n = 42) and the other placebo (n = 42). Efficacy measures included changes in total fat mass, total muscle mass, serum triglyceride, total cholesterol, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), apolipoprotein (apo)A1, and apoB levels. The Wessely and Powell scale was used to evaluate physical and mental fatigue. Subjects were assessed at the beginning and end of the study period. Results: At the end of the study, compared with placebo, the levocarnitine-treated patients showed significant improvements in the following parameters: total fat mass (-3.1 vs -0.5 kg), total muscle mass (+2.1 vs +0.2 kg), total cholesterol (-1.2 vs +0.1 mmol/L), LDL-C (-1.1 vs -0.2 mmol/L), HDL-C (+0.2 vs +0.01 mmol/L), triglycerides (-0.3 vs 0.0 mmol/L), apoA1 (-0.2 vs 0.0 g/L), and apoB (-0.3 vs -0.1 g/L). Wessely and Powell scores decreased significantly by 40% (physical fatigue) and 45% (mental fatigue) in subjects taking levocarnitine, compared with 11% and 8%, respectively, in the placebo group (p < 0.001 vs placebo for both parameters). No adverse events were reported in any treatment group. Conclusion: Administration of levocarnitine to healthy elderly subjects resulted in a reduction of total fat mass, an increase of total muscle mass, and appeared to exert a favourable effect on fatigue and serum lipids.
Takashima H, Maruyama T, Abe M. Significance of Levocarnitine Treatment in Dialysis Patients. Nutrients. 2021 Apr 7;13(4):1219. doi: 10.3390/nu13041219.
Abstract. Carnitine is a naturally occurring amino acid derivative that is involved in the transport of long-chain fatty acids to the mitochondrial matrix. There, these substrates undergo β-oxidation, producing energy. The major sources of carnitine are dietary intake, although carnitine is also endogenously synthesized in the liver and kidney. However, in patients on dialysis, serum carnitine levels progressively fall due to restricted dietary intake and deprivation of endogenous synthesis in the kidney. Furthermore, serum-free carnitine is removed by hemodialysis treatment because the molecular weight of carnitine is small (161 Da) and its protein binding rates are very low. Therefore, the dialysis procedure is a major cause of carnitine deficiency in patients undergoing hemodialysis. This deficiency may contribute to several clinical disorders in such patients. Symptoms of dialysis-related carnitine deficiency include erythropoiesis-stimulating agent-resistant anemia, myopathy, muscle weakness, and intradialytic muscle cramps and hypotension. However, levocarnitine administration might replenish the free carnitine and help to increase carnitine levels in muscle. This article reviews the previous research into levocarnitine therapy in patients on maintenance dialysis for the treatment of renal anemia, cardiac dysfunction, dyslipidemia, and muscle and dialytic symptoms, and it examines the efficacy of the therapeutic approach and related issues.
Pekala J, Patkowska-Sokoła B, Bodkowski R, Jamroz D, Nowakowski P, Lochyński S, Librowski T. L-carnitine--metabolic functions and meaning in humans life. Curr Drug Metab. 2011 Sep;12(7):667-78. doi: 10.2174/138920011796504536.
Abstract. L-Carnitine is an endogenous molecule involved in fatty acid metabolism, biosynthesized within the human body using amino acids: L-lysine and L-methionine, as substrates. L-Carnitine can also be found in many foods, but red meats, such as beef and lamb, are the best choices for adding carnitine into the diet. Good carnitine sources also include fish, poultry and milk. Essentially, L-carnitine transports the chains of fatty acids into the mitochondrial matrix, thus allowing the cells to break down fat and get energy from the stored fat reserves. Recent studies have started to shed light on the beneficial effects of L-carnitine when used in various clinical therapies. Because L-carnitine and its esters help reduce oxidative stress, they have been proposed as a treatment for many conditions, i.e. heart failure, angina and weight loss. For other conditions, such as fatigue or improving exercise performance, L-carnitine appears safe but does not seem to have a significant effect. The presented review of the literature suggests that continued studies are required before L-carnitine administration could be recommended as a routine procedure in the noted disorders. Further research is warranted in order to evaluate the biochemical, pharmacological, and physiological determinants of the response to carnitine supplementation, as well as to determine the potential benefits of carnitine supplements in selected categories of individuals who do not have fatty acid oxidation defects.
Mao CY, Lu HB, Kong N, Li JY, Liu M, Yang CY, Yang P. Levocarnitine protects H9c2 rat cardiomyocytes from H2O2-induced mitochondrial dysfunction and apoptosis. Int J Med Sci. 2014 Aug 15;11(11):1107-15. doi: 10.7150/ijms.9153.
Abstract. Background: Although the protective effects of levocarnitine in patients with ischemic heart disease are related to the attenuation of oxidative stress injury, the exact mechanisms involved have yet to be fully understood. Our aim was to investigate the potential protective effects of levocarnitine pretreatment against oxidative stress in rat H9c2 cardiomyocytes. Methods: Cardiomyocytes were exposed to H2O2 to create an oxidative stress model. The cells were pretreated with 50, 100, or 200 μM levocarnitine for 1 hour before H2O2 exposure. Results: H2O2 exposure led to significant activation of oxidative stress in the cells, characterized by reduced viability, increased intracellular reactive oxygen species, lipid peroxidation, and reduced intracellular antioxidant activity. Mitochondrial dysfunction was also observed following H2O2 exposure, reflected by the loss of mitochondrial transmembrane potential and intracellular adenosine triphosphate. These pathophysiological processes led to cardiomyocyte apoptosis through activation of the intrinsic apoptotic pathway. More importantly, the levocarnitine pretreatment attenuated the H2O2-induced oxidative injury significantly, preserved mitochondrial function, and partially prevented cardiomyocyte apoptosis during the oxidative stress reaction. Western blotting analyses suggested that levocarnitine pretreatment increased plasma protein levels of Bcl-2, reduced Bax, and attenuated cytochrome C leakage from the mitochondria in the cells. Conclusion: Our in vitro study indicated that levocarnitine pretreatment may protect cardiomyocytes from oxidative stress-related damage.
Jones AE, Puskarich MA, Shapiro NI, Guirgis FW, Runyon M, Adams JY, Sherwin R, Arnold R, Roberts BW, Kurz MC, Wang HE, Kline JA, Courtney DM, Trzeciak S, Sterling SA, Nandi U, Patki D, Viele K. Effect of Levocarnitine vs Placebo as an Adjunctive Treatment for Septic Shock: The Rapid Administration of Carnitine in Sepsis (RACE) Randomized Clinical Trial. JAMA Netw Open. 2018 Dec 7;1(8):e186076. doi: 10.1001/jamanetworkopen.2018.6076.
Abstract Importance: Sepsis induces profound metabolic derangements, while exogenous levocarnitine mitigates metabolic dysfunction by enhancing glucose and lactate oxidation and increasing fatty acid shuttling. Previous trials in sepsis suggest beneficial effects of levocarnitine on patient-centered outcomes. Objectives: To test the hypothesis that levocarnitine reduces cumulative organ failure in patients with septic shock at 48 hours and, if present, to estimate the probability that the most efficacious dose will decrease 28-day mortality in a pivotal phase 3 clinical trial. Design, setting, and participants: Multicenter adaptive, randomized, blinded, dose-finding, phase 2 clinical trial (Rapid Administration of Carnitine in Sepsis [RACE]). The setting was 16 urban US medical centers. Participants were patients aged 18 years or older admitted from March 5, 2013, to February 5, 2018, with septic shock and moderate organ dysfunction. Interventions: Within 24 hours of identification, patients were assigned to 1 of the following 4 treatments: low (6 g), medium (12 g), or high (18 g) doses of levocarnitine or an equivalent volume of saline placebo administered as a 12-hour infusion. Main outcomes and measures: The primary outcome required, first, a greater than 90% posterior probability that the most promising levocarnitine dose decreases the Sequential Organ Failure Assessment (SOFA) score at 48 hours and, second (given having met the first condition), at least a 30% predictive probability of success in reducing 28-day mortality in a subsequent traditional superiority trial to test efficacy. Results: Of the 250 enrolled participants (mean [SD] age, 61.7 [14.8] years; 56.8% male), 35, 34, and 106 patients were adaptively randomized to the low, medium, and high levocarnitine doses, respectively, while 75 patients were randomized to placebo. In the intent-to-treat analysis, the fitted mean (SD) changes in the SOFA score for the low, medium, and high levocarnitine groups were -1.27 (0.49), -1.66 (0.38), and -1.97 (0.32), respectively, vs -1.63 (0.35) in the placebo group. The posterior probability that the 18-g dose is superior to placebo was 0.78, which did not meet the a priori threshold of 0.90. Mortality at 28 days was 45.9% (34 of 74) in the placebo group compared with 43.3% (45 of 104) for the most promising levocarnitine dose (18 g). Similar findings were noted in the per-protocol analysis. Conclusions and relevance: In this dose-finding, phase 2 adaptive randomized trial, the most efficacious dose of levocarnitine (18 g) did not meaningfully reduce cumulative organ failure at 48 hours.
Bremer J. Carnitine—metabolism and functions. Physiol. Rev. 1983;63:1420–80.
Jacob C, Belleville F. L-carnitine: metabolism, functions and value in pathology. Pathol Biol (Paris). 1992 Nov;40(9):910-9.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2025-01-04 16:20:56 | Chemical Risk: |

