![]() Transcutol
Transcutol
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
0 pts from Al222
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Transcutol by Al222 (20710 pt) | 2025-Feb-02 18:44 |
| Read the full Tiiip | (Send your comment) |
Transcutol is a skin penetration enhancer, often used in the cosmetic and pharmaceutical industries. It is a clear, colorless liquid that helps improve the absorption and effectiveness of active ingredients in skincare formulations by enhancing their ability to penetrate the skin. Transcutol is a highly purified form of diethylene glycol monoethyl ether, known for its ability to facilitate the delivery of hydrophilic (water-soluble) and lipophilic (fat-soluble) active ingredients into the skin.
Chemical Composition and Structure
Transcutol is primarily composed of:
- Diethylene Glycol Monoethyl Ether (DEGEE): This compound acts as a solvent and penetration enhancer.
- Alcohol Groups: The ether group in the structure helps Transcutol to solvate and transport active ingredients into the skin.
Physical Properties
- Appearance: Clear, colorless liquid.
- Odor: Mild, almost odorless.
- Solubility: Soluble in water, alcohol, and most organic solvents.
- Stability: Stable under normal conditions of use, though sensitive to extreme heat or prolonged exposure to air.
Production Process
- Synthesis: Transcutol is produced through a chemical reaction involving diethylene glycol and ethylene oxide, followed by purification to remove any impurities.
- Purification: The compound is refined to remove water and other impurities, ensuring a high-quality product that meets regulatory standards.
- Standardization: The final product is standardized to ensure consistent levels of purity and effectiveness.
Applications
Medical Applications
- Drug Delivery: Used in pharmaceutical formulations to improve the absorption of active ingredients, particularly in topical medications and transdermal drug delivery systems.
- Skin Penetration Enhancement: Helps enhance the penetration of certain drugs through the skin, improving their therapeutic effects.
Cosmetics
- Skin Penetration Enhancer: Often used in cosmetic formulations, such as creams, serums, and lotions, to improve the delivery of active ingredients into the skin.
- Moisturizing: Transcutol aids in the retention of moisture in the skin by enhancing the penetration of hydrating agents.
- Anti-aging: Used in anti-aging products to help increase the efficacy of anti-wrinkle or firming agents.
Industrial Applications
- Cosmetic Formulations: Commonly used in high-performance skincare products, particularly those containing vitamins, antioxidants, and peptides, to improve their bioavailability.
- Topical Pharmaceutical Products: Found in topical solutions, gels, and creams that require enhanced delivery of the active ingredient.
Environmental and Safety Considerations
- Biodegradability: Transcutol is biodegradable and considered environmentally friendly when used in the correct concentrations.
- Safety Profile: Generally regarded as safe for topical use in regulated concentrations. However, care should be taken to avoid excessive use in formulations, as it may cause skin irritation in some cases.
- Sustainability: It is important to source Transcutol from manufacturers that adhere to sustainable production practices.
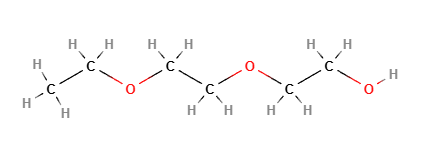 |  |
Molecular Formula C6H14O3
Molecular Weight 134.17 g/mol
CAS 111-90-0
UNII A1A1I8X02B
EC Number 203-919-7
DTXSID2021941
Synonyms:
Diethylene glycol monoethyl ether
2-(2-Ethoxyethoxy)ethanol
Bibliografia__________________________________________________________________________
Mura S, Manconi M, Valenti D, Sinico C, Vila AO, Fadda AM. Transcutol containing vesicles for topical delivery of minoxidil. J Drug Target. 2011 Apr;19(3):189-96. doi: 10.3109/1061186X.2010.483516.
Abstract. The aim of this work was to evaluate the ability of Transcutol (Trc) to produce elastic vesicles with soy lecithin (SL) and study the influence of the obtained vesicles on in vitro (trans)dermal delivery of minoxidil. To this purpose, so-called penetration enhancer-containing vesicles (PEVs) were prepared using Trc aqueous solutions (5-10-20-30% v/v) as hydrophilic phase. SL liposomes, without Trc, were used as control. Prepared formulations were characterized in terms of size distribution, morphology, zeta potential, deformability, and rheological behavior. The influence of the obtained PEVs on (trans)dermal delivery of minoxidil was studied by in vitro diffusion experiments through pig skin. Results showed that all prepared PEVs were able to give good entrapment efficiency (E%≈67) similar to that of conventional liposomes. Trc-containing PEVs showed to be more deformable than liposomes only when minoxidil was loaded in 5 and 10% Trc-containing vesicles. Rheological studies showed that PEVs have higher fluidity than conventional liposomes. All PEVs showed a higher stability than liposomes as shown by studying zeta potential and size distribution during three months. Results of in vitro diffusion experiments showed that Trc-containing PEVs are able to deliver minoxidil to deep skin layers without any transdermal permeation.
Osborne DW, Musakhanian J. Skin Penetration and Permeation Properties of Transcutol®-Neat or Diluted Mixtures. AAPS PharmSciTech. 2018 Nov;19(8):3512-3533. doi: 10.1208/s12249-018-1196-8.
Abstract. A heightened interest in (trans)dermal delivery is in part driven by the need to improve the existing skin therapies and also the demand for alternative routes of administration, notably for pharmaceutical actives with undesirable oral absorption characteristics. The premise of delivering difficult actives to the skin or via the skin however is weighed down by the barrier function properties of the stratum corneum. Short of disrupting the skin by physical means, scientists have resorted to formulation with excipients known to enhance the skin penetration and permeation of drugs. A vehicle that has emerged over the years as a safe solubilizer and enhancer for a broad range of drug actives is the highly purified NF/EP grade of diethylene glycol monoethyl ether (DEGEE) commercially known as Transcutol®. Whereas numerous studies affirm its enhancing effect on drug solubilization, percutaneous absorption rate, and/or drug retention in the skin, there are few publications that unite the body of the published literature in describing the precise role and mechanisms of action for Transcutol®. In view of the current mechanistic understanding of skin barrier properties, this paper takes on a retrospective review of the published works and critically evaluates the data for potential misses due to experimental variables such as formulation design, skin model, skin hydration levels, and drug properties. The goal of this review is to mitigate the incongruence of the published works and to construct a unified, comprehensive understanding of how Transcutol® influences skin penetration and permeation. Graphical Abstract Transcutol has affinity for the hydrophilic head groups of the stratum corneum structures.
Harrison JE, Watkinson AC, Green DM, Hadgraft J, Brain K. The relative effect of Azone and Transcutol on permeant diffusivity and solubility in human stratum corneum. Pharm Res. 1996 Apr;13(4):542-6. doi: 10.1023/a:1016037803128.
Abstract. Purpose: The purpose of this work was to analyse the mechanism of the enhancement of percutaneous penetration demonstrated by the known enhancers Azone and Transcutol. Methods: Enhancer induced changes in the diffusivity and solubility of a model permeant (4-cyanophenol) in human stratum corneum were monitored (in-vitro) using Attenuated total reflectance Fourier transform infra-red (ATR-FTIR) spectroscopy and compared to the gross effects of the enhancers on flux as measured using simple Franz-type diffusion cells. Conclusions: There is increasing interest in the apparently synergistic nature in which certain enhancers appear to work. The exact nature of these multiplicative and/or additive effects is not known although there are numerous suggestions in the current literature. The application of ATR-FTIR spectroscopy to such enhancing systems will allow the mechanisms of the observed enhancements to be probed in greater depth.
Mura P, Faucci MT, Bramanti G, Corti P. Evaluation of transcutol as a clonazepam transdermal permeation enhancer from hydrophilic gel formulations. Eur J Pharm Sci. 2000 Feb;9(4):365-72. doi: 10.1016/s0928-0987(99)00075-5.
Abstract. The influence of diethyleneglycol monoethyl ether (transcutol), alone or in combination with propylene glycol, on clonazepam permeation through an artificial membrane and excised rabbit ear skin from Carbopol hydrogels was investigated. Drug kinetic permeation parameters were determined for both series of experiments and compared. Rheological characteristics, drug solubility and membrane/vehicle partition coefficient for each gel formulation were also determined, and their role in the formulation performance was investigated. Both series of experiments showed an increase of drug permeation as a function of transcutol content in the formulation. The combination of transcutol and propylene glycol resulted in a synergistic enhancement of clonazepam flux. A different trend was found in experiments with gels containing mixtures of the two enhancers, where an increase (in the case of artificial membrane) or a decrease (in the case of rabbit ear skin) of drug permeation was found by increasing the transcutol/propylene glycol ratio in the mixture. Such a result is explained on the basis of the particular mechanism of action demonstrated for transcutol which associates the increase of drug solubility to the potent effect of a depot in the skin.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2025-02-02 18:31:59 | Chemical Risk: |

