![]() Clobetasol propionate
Clobetasol propionate
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Pros:
Antipsoriatic (1)Cons:
Take only under medical supervision (1)0 pts from AColumn
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Clobetasol propionate by AColumn (9336 pt) | 2025-Feb-04 16:11 |
| Read the full Tiiip | (Send your comment) |
Clobetasol Propionate is a potent synthetic corticosteroid used primarily in dermatology for its anti-inflammatory, anti-pruritic, and immunosuppressive effects. It is commonly prescribed for the treatment of inflammatory skin conditions such as eczema, psoriasis, and dermatitis. Clobetasol Propionate works by reducing inflammation and suppressing immune responses in the skin, providing relief from symptoms such as itching, swelling, and redness.
Chemical Composition and Structure
Clobetasol Propionate is a halogenated corticosteroid and its structure is composed of a corticosteroid backbone with the following features:
- Corticosteroid Backbone: The core structure includes the characteristic four-ring structure of corticosteroids, which is essential for its anti-inflammatory and immunosuppressive properties.
- Propionate Ester Group: The ester group (propionate) is attached to the corticosteroid molecule to enhance its potency and skin penetration.
- Halogenation: The presence of chlorine and fluorine atoms in the structure increases its potency compared to other corticosteroids.
Physical Properties
- Appearance: Clobetasol Propionate is typically found as a white to off-white powder or crystalline substance.
- Solubility: It is poorly soluble in water but soluble in alcohol and oils.
- Melting Point: The melting point is typically in the range of 250–270°C, depending on the form.
- Stability: Clobetasol Propionate is stable in its pharmaceutical formulations, though it can degrade under extreme conditions of temperature and light.
Production Process
- Synthesis: Clobetasol Propionate is synthesized through a series of chemical reactions starting with steroid precursors. The process includes halogenation and esterification to form the active compound.
- Purification: The compound is purified to ensure a high concentration of the active ingredient with minimal impurities.
- Formulation: Clobetasol Propionate is incorporated into various formulations such as creams, ointments, lotions, and gels for topical application.
Applications
Medical Applications
- Topical Steroid for Skin Inflammation: Clobetasol Propionate is used to treat inflammatory skin conditions such as psoriasis, eczema, dermatitis, and other allergic or immune-mediated skin disorders.
- Pruritus Treatment: It is effective in relieving itching (pruritus) associated with various dermatologic conditions.
- Autoimmune Skin Disorders: It is used for conditions where the immune system erroneously attacks the skin, such as in autoimmune dermatitis or lupus erythematosus.
Cosmetics
- Skin Repair: In some cosmetic applications, Clobetasol Propionate is used for its potent anti-inflammatory effects in sensitive skin treatments.
- Post-surgical Care: It may be used in post-surgical or post-procedure skin care to reduce inflammation and promote healing.
Cosmetic Safety
Restricted cosmetic ingredient as II / 300 a Relevant Item in the Annexes of the European Cosmetics Regulation 1223/2009. Substance or ingredient reported: Glucocorticoids (Corticosteroids)
Industrial Applications
- Topical Pharmaceuticals: Clobetasol Propionate is primarily found in topical formulations, including prescription creams, gels, and ointments used in clinical settings for skin inflammation.
- Veterinary Use: It may also be used in veterinary medicine for treating inflammatory skin conditions in animals under veterinary supervision.
Environmental and Safety Considerations
- Biodegradability: Clobetasol Propionate is not considered biodegradable, and its environmental impact is a concern when disposed of improperly. Its use should be controlled to prevent environmental contamination.
- Safety Profile: Due to its potency, Clobetasol Propionate is typically used for short periods and under medical supervision. Prolonged use can lead to side effects, including skin thinning, systemic absorption, and potential adrenal suppression.
- Sustainability: The production of Clobetasol Propionate is a synthetic process, and while it is not derived from natural resources, sustainability concerns focus on its production and disposal.
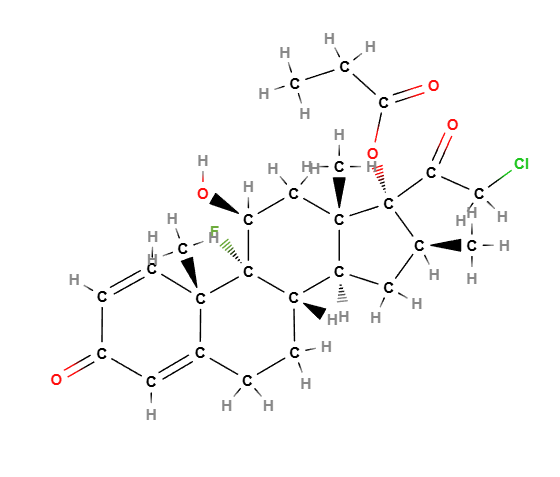 |  |
Molecular Formula C25H32ClFO5
Molecular Weight 467.0 g/mol
CAS 25122-46-7
UNII 779619577M
EC Number 246-634-3
DTXSID6045907
Synonyms:
Clobetasol-17-propionate
Clobex
Dermovate
References__________________________________________________________________________
Menter MA, Caveney SW, Gottschalk RW. Impact of clobetasol propionate 0.05% spray on health-related quality of life in patients with plaque psoriasis. J Drugs Dermatol. 2012 Nov;11(11):1348-54.
Abstract. Psoriasis causes significant distress and impairment in health-related quality of life (QOL) in afflicted patients. For this reason, QOL is an essential and important measure of treatment outcome in patients with the disease. Clobetasol propionate is a super-highpotent class I topical corticosteroid. The spray formulation is approved for twice-daily use for up to 4 weeks by patients 18 years and older with moderate to severe plaque psoriasis. Data collected from 2,236 patients enrolled in 5 clinical trials demonstrate consistent improvement in QOL measures using multiple instruments. In a randomized, double-blind trial in patients with scalp psoriasis, treatment with clobetasol propionate 0.05% spray produced significantly greater improvement in QOL compared with vehicle, as measured by the Scalpdex QOL instrument. In another randomized trial in patients with moderate to severe plaque psoriasis, clobetasol propionate 0.05% spray produced significantly greater reductions in mean affected body surface area and significantly greater improvements in QOL, as measured by the Dermatology Life Quality Index (DLQI), compared with a 0.05% foam formulation. When compared with calcipotriene/betamethasone dipropionate ointment, clobetasol propionate 0.05% spray produced greater rates of lesion clearance and similar improvement in QOL scores after 2 or 4 weeks of treatment. When clobetasol propionate 0.05% spray was used as monotherapy or as an add-on therapy for 4 weeks in a large, observational trial, approximately 80% of patients experienced consistent and significant improvement in QOL on 2 separate, validated QOL instruments (DLQI and the Koo-Menter Psoriasis Index). In conclusion, clobetasol propionate 0.05% spray is an efficacious and safe treatment for plaque psoriasis and produces significant improvement in QOL for affected patients.
Warino L, Balkrishnan R, Feldman SR. Clobetasol propionate for psoriasis: are ointments really more potent? J Drugs Dermatol. 2006 Jun;5(6):527-32.
Abstract. Background: Clobetasol propionate is the most common topical therapy used for psoriasis in the US. Conventional dermatologic wisdom is that ointment preparations provide the highest potency (due to their occlusive nature and moisturizing ability) and are best suited for psoriasis. However, patients often find application of ointment to be messy, raising concerns about both short-term and long-term adherence to treatment. This article reviews the current literature and assesses the relative potency of clobetasol propionate ointment compared to other clobetasol propionate preparations in the treatment of psoriasis. Relevant literature was identified by PubMed and Google searches. We included studies of psoriasis that reported the percentage of subjects that achieved desired efficacy endpoints, as well as studies that reported the subjects' mean change in symptoms from baseline. We excluded studies conducted before 1980 and those that allowed concomitant treatments. Observations: Efficacy rates ranged from 17% to 80% for the different vehicles: ointment, solution, foam, cream, lotion, shampoo, and emollient. Conclusions: Clobetasol propionate is a very effective treatment for psoriasis. Ointment preparations have similar efficacy to other preparations in clinical trial situations. In clinical practice, a situation in which patient preferences are more likely to affect compliance, it may be best to choose whichever vehicle patients find preferable.
Olsen EA, Cornell RC. Topical clobetasol-17-propionate: review of its clinical efficacy and safety. J Am Acad Dermatol. 1986 Aug;15(2 Pt 1):246-55. doi: 10.1016/s0190-9622(86)70164-3.
Abstract. Clobetasol-17-propionate, the most potent of currently available topical steroids as predicted by the vasoconstrictor assay, has just been approved in the United States. In psoriasis, it has proved significantly more effective than class II steroids and as or more effective than the only marketed class I steroid. In the more steroid-responsive eczemas, the superior efficacy of clobetasol is also apparent, but less striking. Clobetasol prolongs remission rates, making intermittent treatment schedules feasible and minimizing inherent potential steroid side effects. Clobetasol may also be useful in the treatment of a myriad of other skin conditions. A review of the pharmacology, efficacy, and side effects of this addition to our dermatologic armamentarium is presented here.
Feldman SR, Yentzer BA. Topical clobetasol propionate in the treatment of psoriasis: a review of newer formulations. Am J Clin Dermatol. 2009;10(6):397-406. doi: 10.2165/11311020-000000000-00000.
Abstract. Ultrapotent topical corticosteroids are the mainstay of psoriasis treatment, used either alone or in combination with a topical vitamin D analog. Traditionally used in an ointment vehicle for psoriasis, clobetasol propionate 0.05% is also available in spray, foam, lotion, and shampoo formulations, which may provide for improved convenience and acceptance in many patients with similar efficacy, safety, and tolerability as the traditional ointment and cream formulations. To compare newer formulations with traditional ointment and cream formulations, we performed a systematic review of the literature. Search terms included 'clobetasol propionate,' in combination with 'psoriasis,' 'vasoconstriction,' 'vasoconstrictor,' or 'absorption' for each of the four vehicles ('spray,' 'foam,' 'lotion,' and 'shampoo'). While there are very few direct comparison studies between clobetasol propionate in different vehicles, the efficacy rates (with success defined as clear or almost clear of psoriasis) for more recent formulations are high, with most patients achieving success after 2-4 weeks of treatment in well controlled clinical trials, with response rates that are similar to those with the traditional clobetasol propionate ointment. Small differences in vasoconstrictor potency or cutaneous absorption have been noted among the formulations, but the clinical significance of these observations is difficult to discern. Recent research has emphasized the importance of treatment adherence in the management of psoriasis. Adherence to treatment is likely to be a far more important determinant of success than are small differences in drug delivery, especially in actual clinical use as opposed to the well controlled environment of clinical trials. For patients who prefer a less messy vehicle, adherence and outcomes are likely to be better with the more recent formulations compared with the traditionally recommended ointment.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Natural Main substances:
Last update: 2025-02-04 15:43:40 | Chemical Risk: |

