![]() Trifluoroacetic acid
Trifluoroacetic acid
Rating : 4
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
0 pts from Al222
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Trifluoroacetic acid by Al222 (20718 pt) | 2025-Apr-24 11:27 |
| Read the full Tiiip | (Send your comment) |
Trifluoroacetic acid (TFA) is a strong organic acid with the chemical formula C₂HF₃O₂. It is a fluorinated carboxylic acid containing three fluorine atoms attached to the carbon backbone. TFA is commonly used in various industries, including pharmaceuticals, biotechnology, and chemistry, due to its unique chemical properties.
Chemical Composition and Structure
TFA is an organofluorine compound with the following structure:
Chemical formula: C₂HF₃O₂
Structure: The structure of TFA consists of a central carbon atom bonded to three fluorine atoms and one carboxyl group (-COOH). The presence of fluorine atoms makes it highly reactive and gives it strong acidic properties.
The presence of fluorine in the molecule enhances the acid strength and makes TFA more volatile than other carboxylic acids, allowing it to be useful in a wide range of applications.
Physical Properties
Appearance: TFA is a colorless, volatile liquid at room temperature.
Odor: It has a pungent, irritating odor.
Solubility: It is highly soluble in water and many organic solvents, such as ethanol, acetone, and chloroform.
Boiling point: TFA has a boiling point of approximately 72.5°C (162.5°F), making it relatively volatile.
pH: As a strong acid, TFA has a very low pH, which makes it highly corrosive to certain materials.
Benefits and Functions
Solvent: TFA is commonly used as a solvent in peptide synthesis and other chemical reactions due to its ability to dissolve a wide range of substances, including proteins and other organic compounds.
Cleavage reagent in peptide synthesis: It is frequently used in peptide synthesis to cleave peptide chains from resin support, as it effectively breaks the bond between the resin and the peptide.
Chemical analysis: TFA is used in the preparation of samples for analytical techniques such as mass spectrometry and chromatography, where its ability to dissolve hydrophobic molecules is beneficial.
Catalyst: In some chemical reactions, TFA acts as a catalyst, promoting certain types of reactions, including esterification and acylation.
Applications
Pharmaceuticals and Biotechnology
Peptide synthesis: TFA is widely used in the synthesis of peptides and proteins, particularly in solid-phase peptide synthesis (SPPS), where it serves as a cleaving agent to remove the peptide from the resin.
Chemical analysis: TFA is often used in the preparation of samples for high-performance liquid chromatography (HPLC) and mass spectrometry (MS) because it helps to improve the solubility of peptides and proteins in these analyses.
Cosmetics
Formulation: TFA is sometimes used in the formulation of cosmetic products, such as exfoliants and chemical peels, due to its strong acidic properties.
Industrial Applications
Cleaning agent: Due to its strong acidity and solubility in organic solvents, TFA is used as a cleaning agent in laboratory settings to remove metal ions, dust, and other contaminants from laboratory equipment.
Environmental and Safety Considerations
Corrosive: TFA is highly corrosive, and it must be handled with care. It can cause severe burns and irritation to the skin, eyes, and respiratory tract.
Toxicity: TFA should be used in well-ventilated areas with appropriate protective equipment, such as gloves, goggles, and lab coats, to minimize the risk of exposure. Ingestion or inhalation of TFA can lead to serious health issues.
Environmental impact: The disposal of TFA should be done carefully, as it can be harmful to the environment if released into water or soil. It should be neutralized before disposal according to safety regulations.
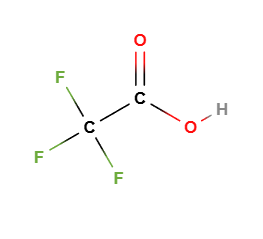 |  |
Molecular Formula C2HF3O2 CF3COOH
Molecular Weight
CAS 76-05-1
UNII E5R8Z4G708
EC Number 200-929-3
DTXSID9041578
Synonyms:
Perfluoroacetic acid
Trifluoroethanoic acid
Bibliografia__________________________________________________________________________
Arp HPH, Gredelj A, Glüge J, Scheringer M, Cousins IT. The Global Threat from the Irreversible Accumulation of Trifluoroacetic Acid (TFA). Environ Sci Technol. 2024 Nov 12;58(45):19925-19935. doi: 10.1021/acs.est.4c06189.
Abstract. Trifluoroacetic acid (TFA) is a persistent and mobile substance that has been increasing in concentration within diverse environmental media, including rain, soils, human serum, plants, plant-based foods, and drinking water. Currently, TFA concentrations are orders of magnitude higher than those of other per- and polyfluoroalkyl substances (PFAS). This accumulation is due to many PFAS having TFA as a transformation product, including several fluorinated gases (F-gases), pesticides, pharmaceuticals, and industrial chemicals, in addition to direct release of industrially produced TFA. Due to TFA's extreme persistence and ongoing emissions, concentrations are increasing irreversibly. What remains less clear are the thresholds where irreversible effects on local or global scales occur. There are indications from mammalian toxicity studies that TFA is toxic to reproduction and that it exhibits liver toxicity. Ecotoxicity data are scarce, with most data being for aquatic systems; fewer data are available for terrestrial plants, where TFA bioaccumulates most readily. Collectively, these trends imply that TFA meets the criteria of a planetary boundary threat for novel entities because of increasing planetary-scale exposure, where potential irreversible disruptive impacts on vital earth system processes could occur. The rational response to this is to instigate binding actions to reduce the emissions of TFA and its many precursors.
Guo Z, Attar AA, Qiqige Q, Lundgren RJ, Joudan S. Photochemical Formation of Trifluoroacetic Acid: Mechanistic Insights into a Fluoxetine-Related Aryl-CF3 Compound. Environ Sci Technol. 2025 Jan 21;59(2):1367-1377. doi: 10.1021/acs.est.4c10777.
Abstract. Trifluoroacetic acid (TFA) is a ubiquitous environmental contaminant; however, its sources are poorly constrained. One understudied source is from the photochemical reactions of aromatic compounds containing -CF3 moieties (aryl-CF3) including many pharmaceuticals and agrochemicals. Here, we studied the aqueous photochemistry of 4-(trifluoromethyl)phenol (4-TFMP), a known transformation product of the pharmaceutical fluoxetine. When exposed to lamps centered at UV-B, 4-TFMP formed up to 9.2% TFA at a steady state under acidic conditions and 1.3% under alkaline conditions. TFA yields of fluoxetine were similar to 4-TFMP for acidic and neutral pH, but higher at alkaline pH, suggesting that fluoxetine may have a mechanism of TFA formation in addition to via the 4-TFMP intermediate. Use of an 13CF3 isotopologue of 4-TFMP allowed for the tracking of TFA formation, which formed via multiple oxidative additions prior to oxidative ring cleavage. The oxidation is mediated by reactive oxygen species (ROS) generated through self-sensitized photolysis, with singlet oxygen and hydroxyl radicals as the key ROS. In addition to the TFA formation mechanism, other photochemical reactions of 4-TFMP resulted in defluorination and dimerization. Overall, this work expands our understanding of how TFA forms from aryl-CF3 compounds to better understand the total global burden of TFA.
Solomon KR, Velders GJ, Wilson SR, Madronich S, Longstreth J, Aucamp PJ, Bornman JF. Sources, fates, toxicity, and risks of trifluoroacetic acid and its salts: Relevance to substances regulated under the Montreal and Kyoto Protocols. J Toxicol Environ Health B Crit Rev. 2016;19(7):289-304. doi: 10.1080/10937404.2016.1175981.
Abstract. Trifluoroacetic acid (TFA) is a breakdown product of several hydrochlorofluorocarbons (HCFC), regulated under the Montreal Protocol (MP), and hydrofluorocarbons (HFC) used mainly as refrigerants. Trifluoroacetic acid is (1) produced naturally and synthetically, (2) used in the chemical industry, and (3) a potential environmental breakdown product of a large number (>1 million) chemicals, including pharmaceuticals, pesticides, and polymers. The contribution of these chemicals to global amounts of TFA is uncertain, in contrast to that from HCFC and HFC regulated under the MP. TFA salts are stable in the environment and accumulate in terminal sinks such as playas, salt lakes, and oceans, where the only process for loss of water is evaporation. Total contribution to existing amounts of TFA in the oceans as a result of the continued use of HCFCs, HFCs, and hydrofluoroolefines (HFOs) up to 2050 is estimated to be a small fraction (<7.5%) of the approximately 0.2 μg acid equivalents/L estimated to be present at the start of the millennium. As an acid or as a salt TFA is low to moderately toxic to a range of organisms. Based on current projections of future use of HCFCs and HFCs, the amount of TFA formed in the troposphere from substances regulated under the MP is too small to be a risk to the health of humans and environment. However, the formation of TFA derived from degradation of HCFC and HFC warrants continued attention, in part because of a long environmental lifetime and due many other potential but highly uncertain sources.
Zhang J, Zhang Y, Li J, Hu J, Ye P, Zeng Z. Monitoring of trifluoroacetic acid concentration in environmental waters in China. Water Res. 2005 Apr;39(7):1331-9. doi: 10.1016/j.watres.2004.12.043.
Abstract. It is critically important and extremely meaningful to determine the concentration of TFA in the environmental water in China. This will create background reference for the effects of analyzing the extensive employment of the substitutes to CFCs in China. In this paper a set of analytical methods was described for use in monitoring of trifluoroacetic acid (TFA) concentration of environmental waters including collecting, pre-treatment measures, preserving, concentrating and derivatization of samples from different kinds of environmental waters. The GC with electrical capture detector (ECD) and headspace auto sampler were used in the analysis. The lowest detection limit of the instrument is 0.0004 ng methyl trifluoroacetic acid (MTFA), and the lowest detected concentration with the method is 3.0 ng/ml TFA. TFA collected in various environmental water samples (including rainfall, inland surface water, ground water, and waste water) from nine provinces and autonomous regions in China have been determined by applying the analytical methods created and defined in this work. The results indicate that the concentrations of TFA in nine rainfalls and three snowfalls through the period from 2000 to 2001 ranged from 25 to 220 ng/l, the TFA concentration in the inland surface water samples ranged from 4.7 to 221 ng/l, the concentration of TFA in groundwater samples collected in Beijing was 10 ng/l, and the TFA concentration in coastal water samples ranged from 4.2 to 190.1 ng/l.
Baqar M, Zhao M, Saleem R, Cheng Z, Fang B, Dong X, Chen H, Yao Y, Sun H. Identification of Emerging Per- and Polyfluoroalkyl Substances (PFAS) in E-waste Recycling Practices and New Precursors for Trifluoroacetic Acid. Environ Sci Technol. 2024 Sep 10;58(36):16153-16163. doi: 10.1021/acs.est.4c05646.
Abstract. Electronic waste is an emerging source of per- and polyfluoroalkyl substance (PFAS) emissions to the environment, yet the contribution from hazardous recycling practices in the South Asian region remains unclear. This study detected 41 PFAS in soil samples from e-waste recycling sites in Pakistan and the total concentrations were 7.43-367 ng/g dry weight (dw) (median: 37.7 ng/g dw). Trifluoroacetic acid (TFA) and 6:2 fluorotelomer sulfonic acid emerged as the dominant PFAS, constituting 49% and 13% of the total PFAS concentrations, respectively. Notably, nine CF3-containing emerging PFAS were identified by the high-resolution mass spectrometry (HRMS)-based screening. Specifically, hexafluoroisopropanol and bistriflimide (NTf2) were consistently identified across all the samples, with quantified concentrations reaching up to 854 and 90 ng/g dw, respectively. This suggests their potential association with electronic manufacturing and recycling processes. Furthermore, except for NTf2, all the identified emerging PFAS were confirmed as precursors of TFA with molar yields of 8.87-40.0% by the TOP assay validation in Milli-Q water. Overall, this study reveals significant emission of PFAS from hazardous e-waste recycling practices and emphasizes the identification of emerging sources of TFA from precursor transformation, which are essential for PFAS risk assessment.
Jubilut GN, Cilli EM, Tominaga M, Miranda A, Okada Y, Nakaie CR. Evaluation of the trifluoromethanosulfonic acid/trifluoroacetic acid/thioanisole cleavage procedure for application in solid-phase peptide synthesis. Chem Pharm Bull (Tokyo). 2001 Sep;49(9):1089-92. doi: 10.1248/cpb.49.1089.
Abstract. As an extension of our investigation of peptidyl-resin linkage stability towards different cleavage procedures used in the solid-phase peptide synthesis (SPPS) technique, the present paper evaluated the trifluoromethanesulfonic acid (TFMSA)/trifluoroacetic acid (TFA)/thioanisole method, varying the type of resin (benzhydrylamine-resin, BHAR; methylbenzhydrylamine-resin, MBHAR and 4-(oxymethyl)-phenylacetamidomethyl-resin, PAMR) and peptide resin-bound residue (Gly and Phe). The vasoactive angiotensin II (AII, DRVYIHPF) and its [Gly8]-AII analogue linked to those resins used routinely in tert-butyloxycarbonyl (Boc)-SPPS chemistry were submitted comparatively to a time course study towards TFMSA/TFA cleavage. At 0 degrees C, [Gly8]-AII was completely removed from all resins in less than 6 h, but the hydrophobic Phe8 moiety-containing AII sequence was only partially cleaved (not more than 15%) from BHAR or MBHAR in this period. At 25 degrees C, [Gly8]-AII cleavage time decreased to less than 2 h irrespective of the solid support, and quantitative removal of AII from PAMR and MBHAR occurred in less than 3 h. However, about 10-15 h seemed to be necessary for cleavage of AII from BHAR, and in this extended cleavage reaction a significant increase in peptide degradation rate was observed. Regardless of the cleavage temperature used, the decreasing order of acid stability measured for resins was BHAR>MBHAR>PAMR. Collectively, these findings demonstrated the feasibility of applying TFMSA/TFA solution as a substitute for anhydrous HF at the cleavage step in Boc-SPPS methodology. Care should be taken however, as the cleavage efficacy depends on multiple factors including the resin, peptide sequence, the time and temperature of reaction.
Valenti LE, Paci MB, De Pauli CP, Giacomelli CE. Infrared study of trifluoroacetic acid unpurified synthetic peptides in aqueous solution: trifluoroacetic acid removal and band assignment. Anal Biochem. 2011 Mar 1;410(1):118-23. doi: 10.1016/j.ab.2010.11.006. Epub 2010 Nov 13. PMID: 21078284.
Zhou J, Saeidi N, Wick LY, Xie Y, Kopinke FD, Georgi A. Efficient removal of trifluoroacetic acid from water using surface-modified activated carbon and electro-assisted desorption. J Hazard Mater. 2022 Aug 15;436:129051. doi: 10.1016/j.jhazmat.2022.129051. Epub 2022 May 14. PMID: 35580494.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances: Last update: 2025-04-24 11:06:32 | Chemical Risk: |

