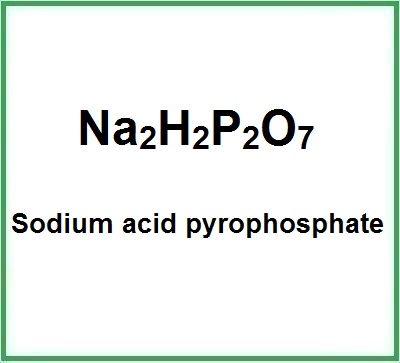
![]() E450 Sodium acid pyrophosphate
E450 Sodium acid pyrophosphate
Rating : 4.8
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
Cons:
To be taken in controlled quantity (1)10 pts from FRanier
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about E450 Sodium acid pyrophosphate Review Consensus 10 by FRanier (9971 pt) | 2024-Oct-05 11:20 |
| Read the full Tiiip | (Send your comment) |
Disodium diphosphate is a chemical compound, anhydrous solid,a linear polymer made up of phosphate units connected to each other by sharing oxygen atoms.
Disodium diphosphate, also known as disodium pyrophosphate, is a salt of pyrophosphoric acid and is commonly used in both food and cosmetic formulations. In cosmetics, it acts as a pH adjuster, stabilizer, and chelating agent, helping to maintain the stability and performance of formulations by binding metal ions and preventing their negative effects on the product. It is often found in personal care products like toothpaste, shampoos, and conditioners, where it helps enhance the product's texture and stability.
Chemical Composition and Structure
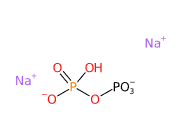
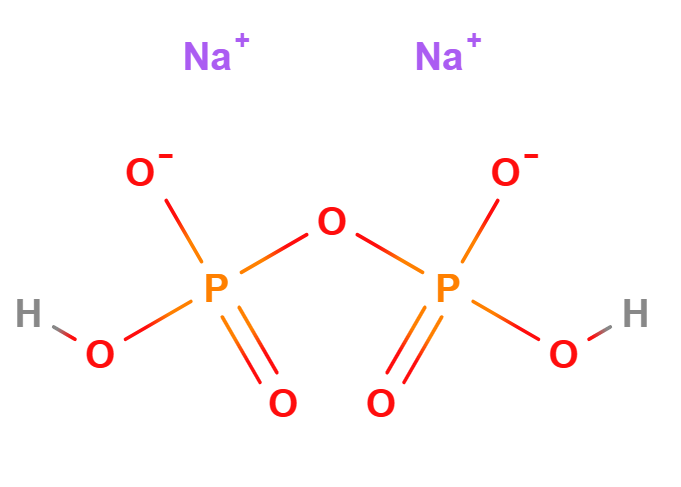
Disodium diphosphate (Na2H2P2O7) consists of two sodium ions and a pyrophosphate anion. Its structure allows it to act as a chelating agent, binding metal ions such as calcium and magnesium, which can interfere with the performance and stability of cosmetic formulations. It also has buffering properties, helping to regulate the pH of formulations.
Physical Properties
Disodium diphosphate typically appears as a white, odorless powder or crystalline solid. It is water-soluble and is easily incorporated into water-based formulations. Its ability to act as a chelating and buffering agent makes it useful in a wide range of cosmetic and personal care products.
The name describes the structure of the molecule:
- 'Di-' is a prefix meaning 'two'. In this case, it refers to the two sodium (Na) atoms in the compound.
- "Sodium" is a chemical element with the symbol Na (from the Latin "natrium").
- The term 'diphosphate' refers to the pyrophosphate anion (P2O74-), which consists of two phosphate groups (PO4) linked by a phosphoanhydride bond.
The synthesis process takes place in different steps:
- Sodium carbonate reacts with phosphoric acid to produce monosodium phosphate, carbon dioxide and water. This is an acid-base reaction in which sodium carbonate (the base) reacts with phosphoric acid (the acid) to produce a salt (monosodium phosphate), water and carbon dioxide.
- When heated, monosodium phosphate undergoes a dehydration reaction, i.e. it loses water. This reaction produces disodium diphosphate. In this reaction, two molecules of monosodium phosphate come together, lose one molecule of water and form a single molecule of disodium diphosphate.
It occurs as a fine or granular, white powder.

It is also called sodium pyrophosphate or sodium acid pyrophosphate, SAPP, etc.and it is composed of sodium, potassium, calcium and phosphates.
What it is used for and where
Food
Ingredient on the European food additives list as E450, emulsifier, raising agent.
The E450 are subdivided into:
- E450(i) Disodium diphosphate
- E450 (ii) Trisodium di phosphate
- E450 (iii) Tetra sodium pyrophosphate
- E450 (v) Tetra potassium pyrophosphate
- E450 (vi) Calcium di hydrogen di phosphate
Used as a leavening agent, sequestering agent, buffering agent, it can be used in tinned food, meat, ham, baking powder and so on.
Cosmetics
- Anticorrosive. Ingredient that prevents and/or inhibits corrosion of packaging material.
- Buffering agent. It is an iingredient that can bring an alkaline or acid solution to a certain pH level and prevent it from changing, in practice a pH stabiliser that can effectively resist instability and pH change.
- Chelating agent. It has the function of preventing unstable reactions and improving the bioavailability of chemical components within a product, and removes calcium and magnesium cations that can cause cloudiness in clear liquids.
Safety
Two studies were conducted in healthy volunteers to confirm the involvement of an excipient, SAPP (sodium acid pyrophosphate), and the mechanism of interaction, altered gastrointestinal transit. Gastrointestinal transit times, determined by scintigraphic imaging, were compared between treatments. Gastric emptying time was unchanged, but small intestinal transit time was decreased to 56% in the presence of SAPP (1).
It is considered a safe component for human health "when they are used at levels that are now underway or it is reasonable to expect in the future" writes the FDA (US Food & Drug Administration) (2) and these levels provide a maximum daily intake of 70 mg / kg of body weight (3).
The effects of two pH levels (5.55 or 5.85) in combination with 0.4% sodium acid pyrophosphate (SAPP), NaH2PO4 X H2O, Na2HPO4 X 7H2O, or NaCl on the growth and toxicity of Clostridium botulinum 52A were studied. Results suggest that the actual production or function of the protease responsible for toxin activation may have been inhibited by the presence of SAPP (4).
Phosphate incorporation into ground beef prior to cooking aids in the reduction of oxidation in the cooked, stored product, although a longer period of time before thermal processing may be necessary for the encapsulated phosphate to have significant benefits (5).
Sodium polyphosphate is a linear polymer formed from phosphate units linked together by sharing oxygen atoms. Addition of calcium to a solution of sodium polyphosphate results in phase separation and formation of a polyphosphate coacervate best described as a polymeric rich viscoelastic material. Polyphosphate coacervate is an interesting candidate as a biomaterial based on its ability to bind with different cations and to be loaded with drugs. Here, in vitro degradation and hemostatic properties of polyphosphate coacervates are comprehensively evaluated. We show that polyphosphate coacervates degrade and dissolve at a fast rate, losing half of their original mass in a week and transforming to mainly pyrophosphate after 4weeks. This burst dissolution phase happens earlier for the coacervate prepared from very short chain polyphosphate but overall using longer polyphosphate chains does not increase the coacervate longevity significantly. Substitution of Ca with Sr or Ba does not affect the hydrolysis of coacervates but slows down their dissolution into the media. In a whole blood clotting assay, coacervates profoundly decrease the clotting time especially when very long chain polyphosphates are used. While coacervate chain length and divalent cation type were found to significantly affect prothrombin time and thromboplastin time compared to the control, no discernible trends were observed. Platelets adhere in large numbers to coacervates, especially those containing long chain polyphosphate, but the cell morphology observed suggests that they might not to be fully activated. Overall, the long chain polyphosphate coacervate holds a great potential as a resorbable hemostatic agent. Divalent cation additions to a sodium polyphosphate solution result in polyphosphate coacervates, or highly viscous gel-like materials, having great potential in bio-applications such as drug delivery and hemostasis. As these coacervates degrade in aqueous environments, we undertook a comprehensive evaluation to better understand the impact of polyphosphate chain length and divalent cation substitution on this hydrolytic response in order to better predict degradation behavior in the body. Furthermore, there is great interest in the role of polyphosphates in hemostasis following recent publications showing that platelets secrete polyphosphates upon thrombin stimulation. In this paper, we evaluate the hemostatic potential of polyphosphate coacervates as bulk constructs, demonstrating that indeed these materials hold great potential as a degradable hemostatic agent (6).
It is an antibacterial agent that has been shown to significantly reduce Escherichia coli, coliforms, and aerobic plate count (APC) on postchill broilers and increased shelflife by 1-2 days when stored at 4.4°C (7).
Typical optimal characteristics of a Disodium diphosphate commercial product
| Appearance | White powder |
| Contenuto Na2H2P2O7 ≥% | 94.72 |
| Anidride fosforica P2O5 ≥% | 61.0 |
| pH di soluzione acquosa all'1% | 3.87 |
| Acqua insolubile % | 0.08 |
| Metalli pesanti ≤% | 0.0008 |
| As ≤% | 0.0001 |
| F ≤% | 0.0007 |
| Pb ≤% | 0.0002 |
| 80mesh ≥% | 96 |
 |  |
 | 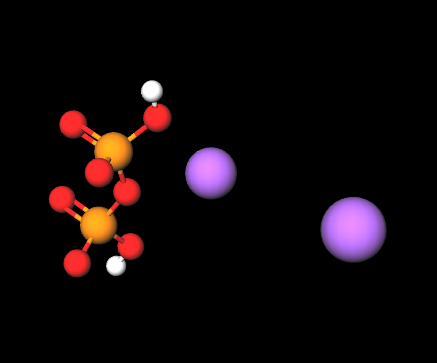 |
- Molecular Formula: Na2H2P2O7 H2Na2O7P2
- Linear Formula Na2H2P2O7
- Molecular Weight: 221.936 g/mol
- CAS: 7758-16-9
- UNII: H5WVD9LZUD
- EC Number: 231-835-0 272-808-3
Synonyms:
Sodium pyrophosphate dibasic
- Disodium dihydrogen pyrophosphate
- Sodium acid pyrophosphate
- Sodium polyphosphate
- Diphosphoric Acid Disodium Salt
- Diphosphoric acid, sodium salt (1:2)
- disodium [hydroxy(oxido)phosphoryl] hydrogen phosphate
- Polyphosphoric acids, sodium salts
- Pyrophosphoric acid, disodium salt
- Dinatriumpyrophosphat
- Sodium polyphosphates
- Grahamsches salz
- Natrium polyphosphat
- Glassy sodium phosphate
- Natrium polymetaphosphat
- disodium pyrophosphate 2-
- Sodium diphosphate dibasic
- Sodium dihydrogen pyrophosphate
- Sodium polyphosphate, amorphous
- sodium dihydrogendiphosphate
- Disodium pytophosphate
- Diphosphoric acid, disodium salt
- Disodium acid pyrophosphate
- Dinatriumpyrophosphat [German]
- Disodium dihydrogen diphosphate
Disodium dihydrogenpyrophosphate
References__________________________________________________________________
(1) Koch KM, Parr AF, Tomlinson JJ, Sandefer EP, Digenis GA, Donn KH, Powell JR Effect of sodium acid pyrophosphate on ranitidine bioavailability and gastrointestinal transit time. - Pharm Res. 1993 Jul
Abstract. During development of a ranitidine effervescent oral solution dosage form, a marked decrease was observed in the extent of ranitidine absorption relative to the conventional oral tablet. Two studies were conducted in healthy volunteers to confirm the involvement of an excipient, SAPP (sodium acid pyrophosphate), and the mechanism of interaction, altered gastrointestinal transit. The first study (n = 12) involved single-dose crossover comparisons of (A) 150 mg ranitidine with 1132 mg SAPP versus (B) 150 mg ranitidine and (C) 150 mg ranitidine with all the effervescent tablet excipients except SAPP versus (D) a 150-mg ranitidine effervescent tablet, all administered as oral solutions. Serum ranitidine AUC, Cmax, and tmax were compared using two one-sided t test 90% confidence intervals (CI). Comparing treatments A to B and D to C, all 90% CI were below the 80-120% range, indicating significantly less extensive ranitidine absorption (54% based on AUC) from the oral solutions containing SAPP. The second study (n = 12) was a single-dose crossover comparing 50 microCi 111 InCl solutions with and without 1132 mg SAPP. Gastrointestinal transit times, determined by scintigraphic imaging, were compared between treatments. Gastric emptying time was unchanged, but small intestinal transit time was decreased to 56% in the presence of SAPP. More rapid small intestinal transit associated with an excipient of a solution dosage form apparently resulted in a decreased extent of ranitidine absorption. This observation contradicts the conventional wisdom that oral solutions are unlikely to fall short of bioequivalence relative to solid oral formulations.
(2) http://www.fda.gov GRAS Substance : 182.1087
(3) Wageningen University, Food-info, E450
(4) Wagner MK, Busta FF. Inhibition of Clostridium botulinum 52A toxicity and protease activity by sodium acid pyrophosphate in media systems. Appl Environ Microbiol. 1985 Jul;50(1):16-20.
Abstract. The effects of two pH levels (5.55 or 5.85) in combination with 0.4% sodium acid pyrophosphate (SAPP), NaH2PO4 X H2O, Na2HPO4 X 7H2O, or NaCl on the growth and toxicity of Clostridium botulinum 52A were studied. Absorbancy measurements at 630 nm, microscopic observations, and the mouse bioassay procedure were used to observe the effects. At pH 5.55 and 5.85 most control cultures exhibited toxicity when cell lysis began. Vegetative cell development was normal (4 micron long; 1 micron wide). SAPP-containing (0.4%) treatment cultures displayed similar growth and lysis but no or delayed (48 h) toxicity. Cells grown in the SAPP treatment culture were longer and wider (6 micron long; 1.5 micron wide) than in most other treatment cultures. Trypsinization of nontoxic supernatants from 0.4% SAPP resulted in toxicity. Addition of 0.4% SAPP to toxic C. botulinum supernatant delayed but did not prevent death of mice. The addition of various levels of SAPP to toxic supernatants resulted in a decrease in zone size with an increase in the level of SAPP (9 mm with 0.4% SAPP to 7 mm with 1.0% SAPP), using a dual substrate protease assay. A decrease in the zone size also occurred with the supernatant from cultures grown in the presence of SAPP and with Bacillus polymyxa protease dilutions containing 0.4% SAPP. Results suggest that the actual production or function of the protease responsible for toxin activation may have been inhibited by the presence of SAPP.
(5) Sickler ML, Claus JR, Marriott NG, Eigel WN, Wang H. Antioxidative effects of encapsulated sodium tripolyphosphate and encapsulated sodium acid pyrophosphate in ground beef patties cooked immediately after antioxidant incorporation and stored. Sickler ML, Claus JR, Marriott NG, Eigel WN, Wang H. Meat Sci. 2013 Jul;94(3):285-8. doi: 10.1016/j.meatsci.2013.03.011. Epub 2013 Mar 16.
(6) Momeni A, Filiaggi MJ Degradation and hemostatic properties of polyphosphate coacervates. Acta Biomater. 2016 Sep 1;41:328-41. doi: 10.1016/j.actbio.2016.06.002. Epub 2016 Jun 2.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (2)
Component type: Chemical Main substances: Last update: 2023-03-21 18:04:15 | Chemical Risk: |