Crospovidone type A (and type B) is a cross polymer consisting of N-vinylpyrrolidone, often referred to as insoluble PVP or cross-linked PVP. Insoluble in water, acid, alkalis and common organic solvents . It is a light yellow or white hygroscopic powder, practically odourless. Insoluble in water, acid and alkali and in all other common solvents.
The name defines the structure of the molecule:
- 'Crospovidone' is a synthetic polymer derived from N-vinyl-2-pyrrolidone (NVP). It is a type of polyvinylpolypyrrolidone (PVPP) that is cross-linked, meaning that the polymer chains are linked to form a three-dimensional network.
- 'Type A' refers to a specific grade or variant of crospovidone. The exact characteristics of Type A crospovidone may vary depending on the manufacturer, but generally refers to a variant that has certain specifications in terms of particle size, pore size and cross-linking density.
The synthesis process takes place in different steps:
- Polymerisation: N-vinyl-2-pyrrolidone (NVP) is polymerised to form polyvinylpyrrolidone (PVP). This is typically done using a free radical polymerisation process, where NVP monomers react in the presence of a free radical initiator.
- Cross-linking: PVP is then cross-linked to form crospovidone. This involves the addition of a cross-linking agent that can react with the PVP chains to form covalent bonds between them. The conditions of the cross-linking reaction, such as the temperature and the amount of cross-linking agent used, can be adjusted to control the degree of cross-linking and the properties of the resulting crospovidone.
- Purification: the resulting crospovidone is purified to remove any unreacted materials and impurities through washing and filtration.
- Drying and milling: the purified crospovidone is dried and milled to the desired particle size. The specific process and milling conditions can be adjusted to produce type A crospovidone with the desired particle size distribution.
- Packaging: The ground crospovidone is then packaged and stored for use in various applications, such as in pharmaceutical formulations where it is used as a disintegrant to help the tablet or capsule separate and release the active ingredient when it comes into contact with water.

Both Crospovidone type A and Crospovidone type B have the characteristic of swelling and leading to the formation of coordination compounds such as polyphenols, carboxylic acid and other low molecular weight compounds. The difference between the two lies in the different density, which leads to sometimes different applications. The higher the density, the finer the powder.
| Crospovidone type A | Density | 1.316 ~ 1.321 g/ml |
| Crospovidone type B | Density | 1.225 ~ 1.342 g/ml |
Tablet compactability. Fine crospovidone grades (type B) increase the tensile strength of tablets at a given compressive pressure. The finer the crospovidone grade, the stronger the bonding capacity.
Tablet compression. Both type A and type B have similar compression values.
Tablet bonding. Copovidone, as a dry binder, increasing the strength of the tablet slows its disintegration. In this case Crospovidone type B can provide rapid disintegration.
What it is used for and where
Medical
By the pharmaceutical industry due to its characteristics of physiological inertness and biological compatibility, it is used as a medical slow-release carrier, disintegration agent, haemodialysis film, etc. Depending on the ingredients in the formula, the disintegration time of capsules and tablets changes.
It acts in different ways:
- Disintegrating in solid tablets due to its ability to absorb water and swelling. Crospovidone is inert and has the same chemical structure as povidone, which is used as a carrier in solid dispersion systems. It improves dissolution and bioavailability.
- Complexing agent for tannin and polyphenols in alcoholic extracts or aqueous herbs.
- Suspension stabiliser increases the dispersion capacity of the solution sediment by acting as a hydrophilic viscosising polymer.
- Absorbs moisture
Crospovidone type A is also incorporated into medicinal tablets as a normal binding agent.
Crospovidone type B increases the tensile strength of tablets at a given value of compressive pressure, and the finer the grade of crospovidone, the greater the binding capacity.
Food
In the beverage industry, beer, fruit juices etc., Crospovidone type A has the property of maintaining taste, removing anthocyanin and polyphenol, improving colour and clarity of the liquid. it also acts as a preservative and stabiliser.
Cosmetics
Crospovidone type A acts as a moisturiser for the skin, maintains the moisture of higher grade cosmetics. In toothpastes it acts as a surfactant and alleviates inflammation.
The most relevant studies on the subject have been selected with a summary of their contents:
Crospovidone studies
| Appearance | White powder |
| pH | 5-7 |
| Boiling Point | 217.6±7.0°C at 760 mmHg |
| Melting Point | ~165 °C |
| Flash Point | 93.9±0.0°C |
| Density | 1.1±0.1 g/cm3 |
| PSA | 20.31000 |
| LogP | 0.37 |
| Vapor Pressure | 0.1±0.4 mmHg at 25°C |
| Refraction Index | 1.593 |
| Soluble components | ≤1.0% |
| Adsorption | >55 g/100g |
Loss on drying
| ≤5.0 % |
Soluble components
| ≤1.0% |
Heavy metals
| ≤10 ppm |
| Nitrogen | 11.0-12.8% |
| Sulfate ash | ≤0.1% |
| Peroxides H2O2 | ≤400 ppm |
Aerobic plate count
| ≤100 cfu/g |
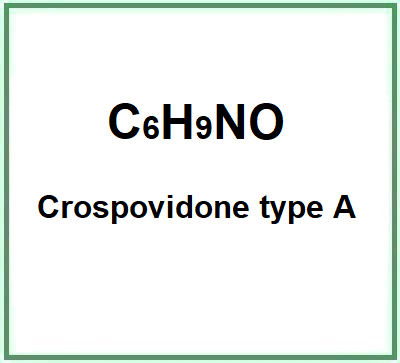
- Molecular Formula C6H9NO C6H13NOP2
- Linear Formula (C6H9NO)n
- Molecular Weight 111.14
- Exact Mass 111.068413
- CAS 25249-54-1 1-Vinyl-2-pyrrolidinone homopolymer (9003-39-8)
- UNII 4LFS24GD0V
- EC Number 201-800-4 607-660-4
- DSSTox Substance ID
- IUPAC 1-Ethenyl-2-pyrrolidinone homopolymer
- InChl=1S/C6H13NOP2/c8-5-2-1-3-7(5)6(10)4-9/h6H,1-4,9-10H2
- InChl Key LQIAZOCLNBBZQK-UHFFFAOYSA-N
- SMILES C1CC(=O)N(C1)C(CP)P
- MDL number MFCD00149016
- PubChem Substance ID
- ChEBI 185135
- RTECS TR8370000
- NCI C80893
- RXCUI 1310706
Synonyms:
- Vinylpyrrolidone
- Polyvinylpyrrolidone
- PVP
- Povidone
- 1-Vinyl-2-pyrrolidinone
- polyvinylpolypyrrolidone
![]() Crospovidone type A
Crospovidone type A