![]() Carboxymethylcellulose Sodium
Carboxymethylcellulose Sodium
Rating : 7
| Evaluation | N. Experts | Evaluation | N. Experts |
|---|---|---|---|
| 1 | 6 | ||
| 2 | 7 | ||
| 3 | 8 | ||
| 4 | 9 | ||
| 5 | 10 |
0 pts from admin
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.Evaluate | Where is this found? |
| "Descrizione" about Carboxymethylcellulose Sodium by admin (19557 pt) | 2025-Jan-04 12:31 |
| Read the full Tiiip | (Send your comment) |
Croscarmellose Sodium, a cross-linked polymer of sodium carboxymethylcellulose, is a multifunctional ingredient widely used in pharmaceutical, cosmetic, and food industries. Known for its exceptional disintegration, water absorption, and swelling properties, it plays a vital role in tablet formulations, topical products, and food stabilization. Its compatibility, safety, and efficacy make it indispensable in diverse applications.
Chemical Composition and Structure
- CAS Number: 9085-26-1
- Molecular Formula: C8H16NaO8
Croscarmellose Sodium is derived from cellulose, a natural polysaccharide. The molecule undergoes functionalization and cross-linking, resulting in the following key features:
- Cellulose Backbone: The linear structure of glucose units in cellulose forms the polymer’s primary framework.
- Carboxymethyl Substitution: Sodium carboxymethyl groups (-CH2COONa) are introduced, enhancing hydrophilicity and enabling rapid water uptake.
- Cross-Linking: Chemical cross-linking stabilizes the polymer, preventing complete solubilization and ensuring structural integrity during swelling.
This design allows Croscarmellose Sodium to exhibit high swelling capacities, making it a superdisintegrant.
Physicochemical Properties
- Appearance: Fine, white to off-white, odorless powder.

- Solubility: Insoluble in water, disperses to form a gel-like matrix upon hydration.
- pH Range: Functions optimally within a pH range of 5.0–7.0, suitable for a wide variety of formulations.
- Swelling Capacity: Absorbs water up to 10-20 times its weight, providing rapid swelling and disintegration.
- Stability:
- Heat-stable under typical processing conditions.
- Resistant to hydrolysis and microbial degradation under appropriate storage.
Production Process
Raw Material Preparation:
- Cellulose is sourced from renewable materials such as wood pulp or cotton linters.
Carboxymethylation:
- Sodium chloroacetate reacts with cellulose in the presence of alkali (sodium hydroxide), introducing carboxymethyl groups.
Cross-Linking:
- A controlled chemical cross-linking step creates a stable, three-dimensional polymer network.
Purification and Drying:
- Residual reactants and impurities are removed through filtration and washing. The product is dried and milled to achieve the desired particle size.
Quality Control:
- The final product undergoes rigorous testing to ensure consistent swelling capacity, particle size distribution, and purity.
Applications
Pharmaceutical Industry
Superdisintegrant:
- Widely used in tablets and capsules to enhance disintegration and dissolution.
- Ensures rapid release of active ingredients, improving drug bioavailability.
Suspension Stabilizer:
- Prevents sedimentation in liquid formulations, maintaining uniformity.
Sustained and Controlled Release Systems:
- Incorporated in formulations requiring delayed release or controlled drug delivery by modifying the polymer network.
Cosmetics Industry
Thickening Agent:
- Adds viscosity to lotions, creams, and gels, providing a smooth application.
Stabilizer in Emulsions:
- Prevents phase separation in oil-in-water and water-in-oil emulsions.
Hydration Enhancer:
- Used in masks and hydrogels to retain water and deliver sustained hydration to the skin.
Exfoliant and Binder:
- Acts as a binder in powder-based cosmetic formulations like masks and exfoliators.
Food Industry
Stabilizer and Thickener:
- Ensures consistency in sauces, gravies, and dressings.
- Provides a desirable texture in baked goods and processed foods.
Dietary Applications:
- Sometimes used as a fiber supplement or texturizing agent in health-conscious formulations.
Functional Mechanisms
Swelling and Disintegration:
- Upon contact with water, Croscarmellose Sodium swells rapidly due to osmotic pressure, breaking apart solid dosage forms.
- The cross-linked network allows high water absorption without dissolution, ensuring structural integrity during swelling.
Hydration and Stabilization:
- In emulsions, the polymer forms a hydrophilic matrix that stabilizes dispersed phases and improves viscosity.
Controlled Release:
- Modified formulations allow the polymer to act as a barrier, regulating the release of active ingredients over time.
Environmental and Safety Considerations
Biodegradability:
- Derived from natural cellulose, it is biodegradable under suitable environmental conditions.
Safety Profile:
- Recognized as Generally Regarded as Safe (GRAS) for pharmaceutical and food applications.
- Non-toxic, hypoallergenic, and suitable for sensitive skin formulations.
Sustainability:
- Renewable sources like wood and cotton contribute to its eco-friendly profile.
- The production process adheres to stringent environmental standards.
Conclusion
Croscarmellose Sodium is a versatile, high-performance polymer that supports modern formulations across diverse industries. Its unique combination of swelling, disintegration, and stabilization properties makes it a cornerstone ingredient in pharmaceuticals, cosmetics, and food products. With its excellent safety and environmental profile, it aligns with the demands for sustainable and efficient solutions in product development.
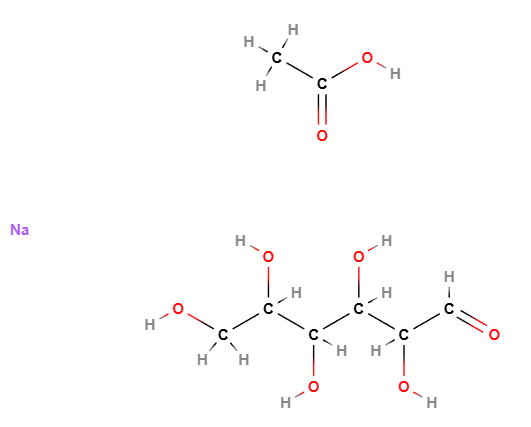 |  |
Molecular Formula C8H16NaO8
Molecular Weight 263.20 g/mol
CAS 9085-26-1
UNII
EC Number 618-378-6
Synonyms:
Carmellose sodium
References__________________________________________________________________________
Zhao N, Augsburger LL. The influence of product brand-to-brand variability on superdisintegrant Performance. A case study with croscarmellose sodium. Pharm Dev Technol. 2006;11(2):179-85. doi: 10.1080/10837450600561281.
Abstract. The purpose of this study is to investigate factors influencing croscarmellose sodium functionality with special emphasis on developing a discriminating model tablet formulation to evaluate product brand-to-brand variability. The particle size distribution, water uptake, and swelling properties of five brands of croscarmellose sodium in either neutral water or 0.1 N HCl were studied. Differences were observed in all properties between brands. Media with acidic pH had a negative impact, but to different extents, on both the water uptake and swelling of all croscarmellose sodium brands due to the presence of carboxymethyl sodium substituents. A tablet matrix composed of lactose (75% w/w) and dicalcium phosphate (25% wt/wt) was used to compare the functional equivalency of the five brands of croscarmellose sodium. The tablet disintegration times were inversely proportional to the swelling ability of superdisintegrant in the testing medium regardless of medium temperature and disintegrant concentration. In conclusion; the particle size, total degree of substitution, and the ratio of basic to acidic substituents are important factors that should be considered during product optimization. The tablet matrix composed of lactose and dicalcium phosphate at a weight ratio of 3:1 can be used as a model formulation for product lot-to-lot consistency and product brand-to-brand comparison purposes.
Gordon MS, Chatterjee B, Chowhan ZT. Effect of the mode of croscarmellose sodium incorporation on tablet dissolution and friability. J Pharm Sci. 1990 Jan;79(1):43-7. doi: 10.1002/jps.2600790111.
Abstract. A computer-optimized experimental design was used to study the effect of incorporating a "super disintegrant", croscarmellose sodium, intragranularly, extragranularly, or distributed equally between the two phases of a tablet in which a poorly soluble drug constituted at least 92.5% of the formulation. The results were analyzed by means of a general quadratic response surface model and suggest that tablets with the same total concentration of super disintegrant dissolve at a faster rate when the super disintegrant is included intragranularly. Tablet friability was not affected by the method of super disintegrant incorporation.
Freeman C, Weiner ML, Kotkoskie LA, Borzelleca J, Butt M. Subchronic and developmental toxicity studies in rats with Ac-Di-Sol croscarmellose sodium. Int J Toxicol. 2003 May-Jun;22(3):149-57. doi: 10.1080/10915810305108.
Abstract. Studies were conducted to evaluate the subchronic and developmental toxicity of Ac-Di-Sol (croscarmellose sodium). In the subchronic study, groups of Sprague-Dawley rats (20/sex/group) received 0 (control), 10000, or 50000 ppm Ac-Di-Sol in the diet for 90 consecutive days (equivalent to 757 and 893 mg/kg/day for males and females fed 10000 ppm, respectively, and to 3922 and 4721 mg/kg/day for males and females fed 50000 ppm, respectively). No mortality, clinical signs of toxicity, or adverse toxicological effects on hematology or serum chemistry parameters, feed consumption, or ophthalmologic examinations were noted in any treatment group. Body weight gain was depressed in high-dose males during the final 3 weeks. The only treatment-related histological lesion noted was moderate renal mineralization at the corticomedullary junction in one high-dose female. This lesion was not considered a specific effect of Ac-Di-Sol, but rather a secondary effect resulting from a potential increase in urinary pH and renal excretion of sodium due to the high intake of sodium associated with Ac-Di-Sol. In the developmental toxicity study, groups of pregnant Sprague-Dawley rats (25/group) received 0 (control), 10000, or 50000 ppm Ac-Di-Sol in the diet on gestational days 6 to 15. No evidence of maternal, fetal, or embryo toxicity was noted. The no-observed-adverse-effect level (NOAEL) for Ac-Di-Sol in both studies exceeds 50000 ppm in the diet, which represents doses of 3922 and 4712 mg/kg/day, for males and females, respectively. The results of these studies demonstrate the low subchronic oral toxicity and developmental toxicity of Ac-Di-Sol, and support the safe use of Ac-Di-Sol in oral applications such as pharmaceuticals, dietary supplements, and sweetener tablets.
| Sign up to vote this object, vote his reviews and to contribute to Tiiips.EvaluateClose | (0 comments) |
Read other Tiiips about this object in __Italiano (1)
Component type: Chemical Main substances:
Last update: 2025-01-04 11:58:42 | Chemical Risk: |

